Abstract
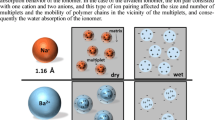
The active water absorption behavior of poly(styrene-co-methacrylate] PSMANa and sulfonated polystyrene PSSNa ionomers was studied. On one hand, the acidic copolymers did not absorb water noticeably. On the other hand, the amount of water absorbed by the ionomer increased with increasing ion content. Especially when the ion contents of the PSMANa and PSSNa ionomers exceeded 6 and 10 mol%, respectively, the maximum amount of the water absorbed by the ionomers increased rapidly as the ion content increased. This indicated that the cluster-dominant ionomers exhibited stronger water absorption behavior, compared to the matrix-dominant ionomers. In addition, when the ion contents of the PSMANa and PSSNa ionomers were less than 6 and 10 mol%, respectively, the volume and shape of the bulk ionomers did not change significantly by the water absorption. Morphological studies on the soaked ionomers showed that the SAXS peak shifted to lower angles as the water uptake increased with increasing ion content of the ionomers, which was consistent with the results obtained by the swelling method.

Similar content being viewed by others
References
A. Eisenberg and J.-S. Kim, Introduction to Ionomer, John Wiley & Sons, New York, 1998.
A. Eisenberg and M. Navratil, Macromolecules, 6, 604 (1973).
M. Escoubes, M. Pineri, S. Gauthier, and A. Eisenberg, J. Appl. Polym. Sci., 29, 1249 (1984).
S. Yano, K. Tadano, N. Nagao, E. Kutsumizu, H. Tachio, and E. Hirasawa, Macromolecules, 25, 7168 (1992).
M. Jiang, A. A. Gronowski, H. L. Yeager, G. Wu, J.-S. Kim, and A. Eisenberg, Macromolecules, 27, 6541 (1994).
H. Tachino, H. Hara, E. Kutsumizu, and S. Yano, J. Appl. Polym. Sci., 55, 131 (1995).
G. Gebel, Polymer, 41, 5829 (2000).
T. D. Gierke, J. Electrochem. Soc., 124, 319 (1977).
M. Falk, Can. J. Chem., 58, 1495 (1980).
S. R. Lowry and K. A. Mauritz, J. Am. Chem. Soc., 102, 4665 (1980).
W. Y. Hsu, J. R. Barley, and P. Meakin, Macromolecules, 13, 198 (1980).
H. I. Yeager and A. Steck, J. Electrochem. Soc., 128, 1880 (1981).
T. D. Gierke, G. E. Munn, and F. C. Wilson, J. Polym. Sci. Polym. Phys. Ed., 19, 1687 (1981).
V. K. Datye, P. L. Taylor, and A. J. Hopfinger, Macromolecules, 17, 1704 (1984).
K. A. Mauritz and C. E. Rogers, Macromolecules, 18, 483 (1985).
P. Aldebert, B. Dreyfus, G. Gebel, N. Nakamura, M. Pineri, and F. Volino, J. Phys. France, 49, 2101 (1988).
K. A. Mauritz and R. B. Moore, Chem. Rev., 104, 4535 (2004) and references therein.
K. Schmidt-Rohr and Q. Chen, Nat. Mater., 7, 75 (2008) and references therein.
C. K. Knox and G. A. Voth, J. Phys. Chem. B, 114, 3205 (2010).
D. Wu, S. J. Paddison, J. A. Elliott, and S. J. Hamrock, Langmuir, 26, 14308 (2010).
J. A. Elliott, D. Wu, S. J. Paddison, and R. B. Moore, Soft Matter, 7, 6820 (2011).
V. Klika, J. Kubant, M. Pavelka, and J. B. Benziger, J. Membr. Sci., 540, 35 (2017).
A. Vishnyakov, R. Mao, M.-T. Lee, and A. V. Neimark, J. Chem. Phys., 148, 024108 (2018).
P. A. Tamirisa and D. W. Hess, Macromolecules, 39, 7092 (2006).
E. Sacher and J. R. Susko, J. Appl. Polym. Sci., 26, 679 (1981).
S. J. Kim, S. R. Shin, S. M. Lee, I. Y. Kim, and S. I. Kim, J. Appl. Polym. Sci., 88, 2721 (2003).
L. A. McDonough, B. Dragnea, J. Preusser, and S. R. Leone, J. Phys. Chem. B., 107, 4951 (2003).
J.-S. Kim, R. J. Jackman, and A. Eisenberg, Macromolecules, 27, 2789 (1994).
H. S. Makowski, R. D. Lundberg, and G. L. Singhal, US Patent 870,841 (1975).
J. A. Lefelar and R. A. Weiss, Macromolecules, 17, 1145 (1984).
B. Hird and A. Eisenberg, Macromolecules, 25, 6466 (1992).
S. N. Kasarova, N. G. Sultanova, C. D. Ivanov, and I. D. Nikolov, Opt. Mater., 29, 1481 (2007).
M. Daimon and A. Masumura, Appl. Optics, 46, 3811 (2007).
S. N. Ege, Organic Chemistry: Structure and Reactivity, 5th ed., Houghton Mifflin Company, College Div., Boston, 2003.
P. Atkins and J. de Paula, Atkins’ Physical Chemistry, 10th ed., Oxford University Press, Oxford, 2014.
M. Fujimura, T. Hashimoto, and H. Kawai, Macromolecules, 15, 136 (1982).
B. Dreyfus, G. Gebel, P. Aldebert, M. Pineri, M. Escoubes, and M. Thomas, J. Phys. France, 51, 1341 (1990).
D. J. Yarusso and S. L. Cooper, Macromolecules, 16, 1871 (1983).
J.-M. Song and J.-S. Kim, J. Nanosci. Nanotechnol., 8, 5454 (2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgment: This study was supported financially by Chosun University (2015 Intramural Research Grant).
Rights and permissions
About this article
Cite this article
So, IS., Kim, JS. Study of Active Water Absorption of Polystyrene-Based Ionomers. Macromol. Res. 28, 932–938 (2020). https://doi.org/10.1007/s13233-020-8129-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13233-020-8129-6