Abstract
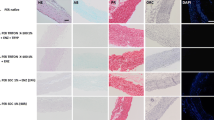
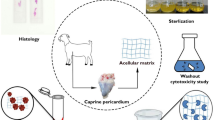
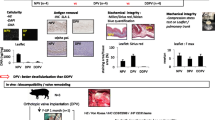
Residual chemicals that are presented during tissue processing in human tissue banks can be a risk for the allograft recipient. Determine the residual concentrations of the antibiotics and detergent used in the process of human decellularized tissue-engineered heart valves stored in isotonic saline solution up to 18 months. A total of 24 human decellularized allografts were stored in sterile sodium chloride and analyzed immediately after the decellularization process (0 months) and after storage for 6, 12, and 18 months, which includes the use of sodium dodecyl sulfate (SDS) and antibiotics (cefoxitin, vancomycin hydrochloride, lincomycin hydrochloride, polymyxin B sulfate). These valves were used for suitability tests, the zone of inhibition evaluation, and direct contact cytotoxicity assay. The stock solution from 32 valves was used for LC–MS/MS analysis of antibiotics and SDS. Tissue samples from decellularized valves showed a zone of inhibition formation for S. aureus and B. subtilis, suggesting the presence of an inhibitory molecule in the tissue. Cytotoxicity tests were negative. Polymyxin B, vancomycin, and SDS were detected and quantified in human decellularized aortic and pulmonary allografts during all periods of the study. There were no traces of residual cefoxitin and lincomycin in the tissue stock solution. We found residual concentrations of the antibiotics and detergent used in the process of human decellularized tissue-engineered heart valves stored in isotonic saline solution up to 18 months.


Similar content being viewed by others
References
Arnold T, Linke D (2007) Phase separation in the isolation and purification of membrane proteins. Biotechniques 43:427–430 passim
Brubaker S, Lotherington K, Zhao J et al (2016) Tissue recovery practices and bioburden: a systematic review. Cell Tissue Bank 17:561–571
Buzzi M, Guarino A, Gatto C et al (2014) Residual antibiotics in decontaminated human cardiovascular tissues intended for transplantation and risk of falsely negative microbiological analyses. PLoS ONE 9:e112679
Caamano S, Shiori A, Strauss SH, Orton EC (2009) Does sodium dodecyl sulfate wash out of detergent-treated bovine pericardium at cytotoxic concentrations? J Heart Valve Dis 18:101–105
Cebotari S, Tudorache I, Jaekel T et al (2010) Detergent decellularization of heart valves for tissue engineering: toxicological effects of residual detergents on human endothelial cells. Artif Organs 34:206–210
Cigliano A, Gandaglia A, Lepedda AJ et al (2012) Fine structure of glycosaminoglycans from fresh and decellularized porcine cardiac valves and pericardium. Biochem Res Int 2012:979351
Crapo PM, Gilbert TW, Badylak SF (2011) An overview of tissue and whole organ decellularization processes. Biomaterials 32:3233–3243
Eastlund T (2006) Bacterial infection transmitted by human tissue allograft transplantation. Cell Tissue Bank 7:147–166
FDA. Pharmaceutical Microbiology Manual. In: Guilfoyle D. US Pharmacopeia, 2014
Germain M, Strong DM, Dowling G et al (2016) Disinfection of human cardiac valve allografts in tissue banking: systematic review report. Cell Tissue Bank 17:593–601
Iop L, Bonetti A, Naso F et al (2014) Decellularized allogeneic heart valves demonstrate self-regeneration potential after a long-term preclinical evaluation. PLoS ONE 9:e99593
ISO. International Organization for Standardization - 10993-5: 2009. Biological evalutation of medical devices, 2009
Jashari R, Faucon F, Hoeck BV, Gelas SD, Fan Y, Vandenbulcke S (2011) Determination of residual antibiotics in cryopreserved heart valve allografts. Transfus Med Hemother 38:379–386
Knight RL, Booth C, Wilcox HE, Fisher J, Ingham E (2005) Tissue engineering of cardiac valves: re-seeding of acellular porcine aortic valve matrices with human mesenchymal progenitor cells. J Heart Valve Dis 14:806–813
Leeming JP, Lovering AM, Hunt CJ (2005) Residual antibiotics in allograft heart valve tissue samples following antibiotic disinfection. J Hosp Infect 60:231–234
Meyer SR, Chiu B, Churchill TA, Zhu L, Lakey JR, Ross DB (2006) Comparison of aortic valve allograft decellularization techniques in the rat. J Biomed Mater Res A 79:254–262
Mirsadraee S, Wilcox HE, Korossis SA et al (2006) Development and characterization of an acellular human pericardial matrix for tissue engineering. Tissue Eng 12:763–773
Paolin A, Romualdi C, Romagnoli L, Trojan D (2017) Analysis of potential factors affecting allografts contamination at retrieval. Cell Tissue Bank 18:539–545
Rieder E, Kasimir MT, Silberhumer G et al (2004) Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J Thorac Cardiovasc Surg 127:399–405
Saegeman V, Ectors N, Lismont D, Verduyckt B, Verhaegen J (2008) Bacteriostasis testing on allograft tissue inoculated in Wilkins-Chalgren broth. J Hosp Infect 70:278–283
Saegeman V, Verhaegen J, Lismont D, Verduyckt B, De Rijdt T, Ectors N (2009) Influence of postmortem time on the outcome of blood cultures among cadaveric tissue donors. Eur J Clin Microbiol Infect Dis 28:161–168
Seddon AM, Curnow P, Booth PJ (2004) Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta 1666:105–117
Tudorache I, Theodoridis K, Baraki H et al (2016) Decellularized aortic allografts versus pulmonary autografts for aortic valve replacement in the growing sheep model: haemodynamic and morphological results at 20 months after implantation. Eur J Cardiothorac Surg 49:1228–1238
USP. Validation of alternative microbiological methods. In: United States Pharmacopeia. 40th ed. Rockville: The United States Pharmacopeial Convention, Inc. 2017
Vafaee T, Thomas D, Desai A et al (2018) Decellularization of human donor aortic and pulmonary valved conduits using low concentration sodium dodecyl sulfate. J Tissue Eng Regen Med 12:e841–e853
Wollmann L, Suss P, Mendonca J et al (2019) Characterization of decellularized human pericardium for tissue engineering and regenerative medicine applications. Arq Bras Cardiol 113:11–17
Acknowledgements
None.
Funding
This work was supported by the Brazilian Ministry of Health (FNS 814611/2014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kraft, L., Ribeiro, V.S.T., de Nazareno Wollmann, L.C.F. et al. Determination of antibiotics and detergent residues in decellularized tissue-engineered heart valves using LC–MS/MS. Cell Tissue Bank 21, 573–584 (2020). https://doi.org/10.1007/s10561-020-09856-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-020-09856-x