Abstract
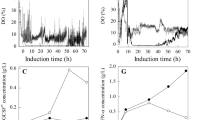
A number of limitations exist for production of human granulocyte colony-stimulating factor (G-CSF) in Pichia pastoris. In this study, two different specific growth rates (0.015 h−1, 0.01 h−1) were used sequentially in the mixed substrate feeding period during methanol induction phase to enhance the G-CSF titer in the culture broth. Necessary parameters required for implementing the feeding strategy, such as specific product yield on biomass (YP/X) and maintenance coefficient (m) on glycerol, methanol, and sorbitol were estimated using continuous culture technique. Using this strategy, for the same volumetric productivity, about 20% increase in protein titer was achieved over that obtained from the run carried out at a single pre-set value of 0.015 h−1 alone. Thus, implementation of higher specific growth rate (0.015 h−1) set during initial stages of the methanol induction phase followed by a lower specific growth rate (0.01 h−1) helped in achieving increased protein titers.




Similar content being viewed by others
References
Cereghino JL, Cregg JM (2000) Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol Rev 24:45–66
Damasceno LM, Huang CJ, Batt CA (2012) Protein secretion in Pichia pastoris and advances in protein production. Appl Microbiol Biotechnol 93:31–39
Langer ES (2012) Biomanufacturing outsourcing outlook. BioPharm Int 25:15–16
Demain AL, Vaishnav P (2009) Production of recombinant proteins by microbes and higher organisms. Biotechnol Adv 27:297–306
Looser V, Bruhlmann B, Bumbak F et al (2015) Cultivation strategies to enhance productivity of Pichia pastoris: a review. Biotechnol Adv 33:1177–1193
Stratton J, Chiruvolu V, Meagher M (1998) High cell-density fermentation. In: Higgins DR, Cregg JM (eds) Methods in molecular biology: Pichia protocols. Humana, Totowa, pp 107–120
Cos O, Ramon R, Montesinos J et al (2006) Operational strategies, monitoring and control of heterologous protein production in the methylotrophic yeast Pichia pastoris under different promoters: a review. Microb Cell Fact 5:17
Cunha AE, Clemente JJ, Gomes R et al (2004) Methanol induction optimization for scFv antibody fragment production in Pichia pastoris. Biotechnol Bioeng 86:458–467
Zhang W, Sinha J, Smith LA et al (2005) Maximization of production of secreted recombinant proteins in Pichia pastoris fed-batch fermentation. Biotechnol Prog 21:386–393
Wu D, Chub J, Hao YY et al (2012) Incomplete protein disulfide bond conformation and decreased protein expression result from high cell growth during heterologous protein expression in Pichia pastoris. J Biotechnol 157:107–112
Mallem M, Warburton S, Li F et al (2014) Maximizing recombinant human serum albumin production in a MutsPichia pastoris strain. Biotechnol Prog 30:1488–1496
Batra J, Beri D, Mishra S (2014) Response surface methodology based optimization of β-glucosidase production from Pichia pastoris. Appl Biochem Biotechnol 172:380–393
Celik E, Calik P, Oliver SG (2009) Fed-batch methanol feeding strategy for recombinant protein production by Pichia pastoris in the presence of co-substrate sorbitol. Yeast 26:473–484
Jungo C, Schenk J, Pasquier M et al (2007) A quantitative analysis of the benefits of mixed feeds of sorbitol and methanol for the production of recombinant avidin with Pichia pastoris. J Biotechnol 131:57–66
Ramón R, Ferrer P, Valero F (2007) Sorbitol co-feeding reduces metabolic burden caused by the overexpression of a Rhizopus oryzae lipase in Pichia pastoris. J Biotechnol 130:39–46
Niu H, Jost L, Pirlot N, Sassi H, Daukandt M, Rodriguez C, Fickers P (2013) A quantitative study of methanol/sorbitol co-feeding process of a Pichia pastoris Mut+/pAOX1-lacZ strain. Microb Cell Fact 12:1–8
Buchetics M, Dragosits M, Maurer M et al (2011) Reverse engineering of protein secretion by uncoupling of cell cycle phases from growth. Biotechnol Bioeng 108:2403–2412
Guba SC, Sartor CA, Hutchinson R et al (1994) Granulocyte colony- stimulating factor (G-CSF) production and G-CSF receptor structure in patients with congenital neutropenia. Blood 83:1486–1492
Crawford J, Ozer H, Stoller R et al (1991) Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Eng J Med 325:164–170
Lasnik MA, Porekar VG, Stalc A (2001) Human granulocyte colony stimulating factor (hG-CSF) expressed by methylotrophic yeast Pichia pastoris. Pflugers Arch Eur J Physiol 442:R184–R186
Bahrami A, Shojaosadati SA, Khalilzadeh R et al (2008) Two-stage glycerol feeding for enhancement of recombinant hG-CSF production in a fed-batch culture of Pichia pastoris. Biotechnol Lett 30:1081–1085
Apte-Deshpande A, Somani S, Mandal G et al (2009) Over expression and analysis of O-glycosylated recombinant human granulocyte colony stimulating factor in Pichia pastoris using Agilent 2100 Bioanalyzer. J Biotechnol 143:44–50
Chien S-F (2010) Cloning and expression of bioactive human granulocyte colony stimulating factor in Pichia pastoris. J Chin Chem Soc 57:850–856
Maity N, Thawani A, Sharma A et al (2016) Expression and control of codon-optimized granulocyte colony-stimulating factor in Pichia pastoris. Appl Biochem Biotechnol 78:159–172
Bai J, Swartz DJ, Protasevich II, Brouillette CG, Harrell PM, Hildebrandt E, Gasser B, Mattanovich D, Ward A, Chang G, Urbatsch IL (2011) A gene optimization strategy that enhances production of fully functional P-glycoprotein in Pichia pastoris. PLoS ONE 3(6):e22577
D’Anjou MC, Daugulis AJ (2001) A rational approach to improving productivity in recombinant Pichia pastoris fermentation. Biotechnol Bioeng 72:1–11
Bok SH, Demain AL (1977) An improved colorimetric assay for polyols. Anal Biochem 81:18–20
Lin-Cereghino J, Lin-Cereghino GP (2007) Vectors and strains for expression. Pichia protocols. Humana Press, Totowa, pp 11–25
Clare JJ, Rayment FB, Ballantyne SP, Sreerkrishna K, Romanos MA (1991) High-level expression of tetanus toxin fragment C in Pichia pastoris strains containing multiple tandem integrations of the gene. Biotechnology 9:455–460
Aw R, Polizzi KM (2016) Liquid PTVA: a faster and cheaper alternative for generating multi-copy clones in Pichia pastoris. Microb Cell Factor 15:29
Trinh LB, Phue JN, Shiloach J (2003) Effect of methanol feeding strategies on production and yield of recombinant mouse endostatin from Pichia pastoris. Biotechnol Bioeng 82:438–444
Näätsaari L, Mistlberger B, Ruth C, Hajek T, Hartner FS, Glieder A (2012) Deletion of the Pichia pastoris KU70 homologue facilitates platform strain generation for gene expression and synthetic biology. PLoS ONE 7:e39720
Hesketh AR, Castrillo JI, Sawyer T et al (2013) Investigating the physiological response of Pichia (Komagataella) pastoris GS115 to the heterologous expression of misfolded proteins using chemostat cultures. Appl Microbiol Biotechnol 97:9747–9762
d'Anjou MC, Daugulis AJ (2000) Mixed-feed exponential feeding for fed-batch culture of recombinant methylotrophic yeast. Biotechnol Lett 22:341–346
Zheng X, Zhang Y, Zhang X, Li C, Liu X, Lin Y, Liang S (2019) Fhl1p protein, a positive transcription factor in Pichia pastoris, enhances the expression of recombinant proteins. Microb Cell Factor 18:1
Whyteside G, Alcocer MJ, Kumita JR et al (2011) Native-state stability determines the extent of degradation relative to secretion of protein variants from Pichia pastoris. PLoS ONE 6:e22692
Kadowaki H, Nishitoh H (2013) Signaling pathways from the endoplasmic reticulum and their roles in disease. Genes 4(3):306–333
Hetz C, Papa FR (2018) The unfolded protein response and cell fate control. Mol Cell 69:169–181
Guerfal M, Ryckaert S, Jacobs PP et al (2010) The HAC1 gene from Pichia pastoris: characterization and effect of its expression on the production of secreted, surface displayed and membrane proteins. Microb Cell Fact 9:49
Valkonen M, Penttila M, Saloheimo M (2003) Effects of inactivation and constitutive expression of the unfolded-protein response pathway on protein production in the yeast Saccharomyces cerevisiae. Appl Environ Microbiol 69:2065–2072
Breinig F, Diehl B, Rau S et al (2006) Cell surface expression of bacterial esterase A by Saccharomyces cerevisiae and its enhancement by constitutive activation of the cellular unfolded protein response. Appl Environ Microbiol 72:7140–7147
Heyland J, Blank LM, Schmid A (2011) Quantification of metabolic limitations during recombinant protein production in Escherichia coli. J Biotechnol 155:178–184
Potgieter TI, Kersey SD, Mallem MR et al (2010) Antibody expression kinetics in glycoengineered Pichia pastoris. Biotechnol Bioeng 106:918–927
Schenk J, Balazs K, Jungo C et al (2008) Influence of specific growth rate on specific productivity and glycosylation of a recombinant avidin produced by a Pichia pastoris Mut+ strain. Biotechnol Bioeng 99:368–377
Jeong KJ, Lee SY (2001) Secretory production of human granulocyte colony-stimulating factor in Escherichia coli. Protein Exp Purif 23:311–318
Rotondaro L, Mazzanti L, Mele A et al (1997) High-level expression of a cDNA for human granulocyte colony-stimulating factor in Chinese hamster ovary cells. Mol Biotechnol 7:231–240
Acknowledgements
This work was supported by a grant from IIT Delhi under the `High impact research and technology leadership project’ (MI00806) awarded to SM and others.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gautam, A., Sahai, V. & Mishra, S. Development of a dual specific growth rate-based fed-batch process for production of recombinant human granulocyte colony-stimulating factor in Pichia pastoris. Bioprocess Biosyst Eng 44, 103–112 (2021). https://doi.org/10.1007/s00449-020-02427-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02427-0