Abstract
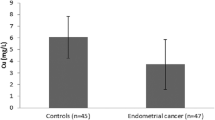
It was aimed to evaluate serum zinc and copper levels and oxidative stress parameters in ectopic pregnancy cases, healthy pregnant women, and healthy non-pregnant women. In this cross-sectional case-control study, 30 patients diagnosed with tubal ectopic pregnancy in the gynecology clinic of a tertiary hospital constituted the study group. A healthy pregnant control group (n = 30) was formed of age, body mass index (BMI), and gestational week-matched subjects, and a healthy non-pregnant control group (n = 30) was formed of age and BMI-matched women. The groups were compared in terms of demographic characteristics and laboratory parameters including serum zinc (Zn) level, serum copper (Cu) level, serum malondialdehyde (MDA) level, serum catalase (CAT) activity, serum glutathione peroxidase (GPX) activity, and serum superoxide dismutase (SOD) activity. The groups were similar in respect of demographic characteristics. In the ectopic pregnancy group, serum GPX activity and Cu level were significantly lower, and serum SOD and CAT activity and Zn and MDA levels were higher compared with those of the healthy pregnant and healthy non-pregnant groups. The Cu/Zn ratio showed a significant, positive correlation with the serum GPX activity and serum progesterone level and a negative correlation with serum SOD and CAT activity. When 1.14 was taken as the cutoff value, sensitivity and specificity of the Cu/Zn ratio to determine ectopic pregnancy were 73.3% and 80.0%, respectively. Comparing the area under curve (AUC) in the ROC (receiver operating characteristic) curve analysis, the Cu/Zn ratio was determined to be more valuable than the Cu or Zn values alone in predicting ectopic pregnancy. In correlation analysis, serum beta hCG level showed a negative correlation with SOD and CAT activities and Zn levels. Serum progesterone level showed a negative correlation with serum CAT and SOD activities and MDA and zinc levels and a positive correlation with serum GPX activity and serum copper level (p < 0.05 for all). The current study can be considered of value as the first study in literature to show a significantly lower serum Zn level and higher serum Cu level in ectopic pregnancy cases compared with healthy pregnant control cases. This is also the first study to have revealed an association between the serum Cu/Zn ratio, oxidative status, and ectopic pregnancy. Furthermore, the serum Cu/Zn ratio was found to be useful in the diagnosis of ectopic pregnancy cases.

Similar content being viewed by others
References
Shaw JL, Dey SK, Critchley HO, Horne AW (2010) Current knowledge of the aetiology of human tubal ectopic pregnancy. Hum Reprod Update 16(4):432–444
Shao R (2010) Understanding the mechanisms of human tubal ectopic pregnancies: new evidence from knockout mouse models. Hum Reprod (Oxford, England) 25(3):584–587
Rana P, Kazmi I, Singh R, Afzal M, Al-Abbasi FA, Aseeri A, Singh R, Khan R, Anwar F (2013) Ectopic pregnancy: a review. Arch Gynecol Obstet 288(4):747–757
Rausch ME, Barnhart KT (2012) Serum biomarkers for detecting ectopic pregnancy. Clin Obstet Gynecol 55(2):418–423
Ashworth CJ, Antipatis C (2001) Micronutrient programming of development throughout gestation. Reproduction (Cambridge, England) 122(4):527–535
Wolonciej M, Milewska E, Roszkowska-Jakimiec W (2016) Trace elements as an activator of antioxidant enzymes. Postepy Hig Med Dosw (Online) 70(0):1483–1498
Hordyjewska A, Popiołek Ł, Kocot J (2014) The many “faces” of copper in medicine and treatment. Biometals 27(4):611–621
Vallee BL, Falchuk KH (1993) The biochemical basis of zinc physiology. Physiol Rev 73(1):79–118
Wilson RL, Grieger JA, Bianco-Miotto T, Roberts CT (2016) Association between maternal zinc status, dietary zinc intake and pregnancy complications: a systematic review. Nutrients 8(10):641
Al-Gubory KH, Fowler PA, Garrel C (2010) The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol 42(10):1634–1650
Lopes AS, Lane M, Thompson JG (2010) Oxygen consumption and ROS production are increased at the time of fertilization and cell cleavage in bovine zygotes. Hum Reprod (Oxford, England) 25(11):2762–2773
Harvey AJ, Kind KL, Thompson JG (2002) REDOX regulation of early embryo development. Reproduction (Cambridge, England) 123(4):479–486
Lu J, Wang Z, Cao J, Chen Y, Dong Y (2018) A novel and compact review on the role of oxidative stress in female reproduction. Reprod Biol Endocrinol : RB&E 16(1):80
Orsi NM, Leese HJ (2001) Protection against reactive oxygen species during mouse preimplantation embryo development: role of EDTA, oxygen tension, catalase, superoxide dismutase and pyruvate. Mol Reprod Dev 59(1):44–53
Perrone S, Laschi E, Buonocore G (2019) Biomarkers of oxidative stress in the fetus and in the newborn. Free Radic Biol Med 142:23–31
Hilali N, Aksoy N, Vural M, Camuzcuoglu H, Taskin A (2013) Oxidative status and serum prolidase activity in tubal ectopic pregnancy. JPMA J Pak Med Assoc 63(2):169–172
SUCU S, BADEMKIRAN MH, ÖZCAN HÇ, KÖMÜRCÜ Ö, BAYRAMOĞLU D, BALAT Ö (2018) Oxidative stress markers in tubal ectopic pregnancy. J Clin Obstet Gynecol 28(1):9–13
Bozkaya G, Karaca I, Fenercioglu O, Yildirim Karaca S, Bilgili S, Uzuncan N (2019) Evaluation of maternal serum ischemia modified albumin and total antioxidant status in ectopic pregnancy. J Matern Fetal Neonatal Med 32(12):2003–2008
Iqbal A, Saeed M, Amjad S (2018) A cross-sectional research: incidence of tubal ectopic pregnancy versus prolidase activity and oxidative stress: one year research experience at Service Hospital, Lahore. Indo Am J Pharm Sci 5(5):4533–4537
Dialani V, Levine D (2004) Ectopic pregnancy: a review. Ultrasound Q 20(3):105–117
Smith JC Jr, Butrimovitz GP, Purdy WC (1979) Direct measurement of zinc in plasma by atomic absorption spectroscopy. Clin Chem 25(8):1487–1491
Evenson MA (1988) Measurement of copper in biological samples by flame or electrothermal atomic absorption spectrometry. Methods Enzymol 158:351–357
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Beutler E (1975) A manual of biochemical methods, New York, pp 62–94
Beutler E (1975) Red cell metabolism 2nd. Grune and Stratton Company, New York, pp 261–265
Fridovich I (1986) Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol 58(6):61–97
Kong BY, Duncan FE, Que EL, Xu Y, Vogt S, O'Halloran TV, Woodruff TK (2015) The inorganic anatomy of the mammalian preimplantation embryo and the requirement of zinc during the first mitotic divisions. Dev Dyn 244(8):935–947
Suzuki T, Yoshida N, Suzuki E, Okuda E, Perry AC (2010) Full-term mouse development by abolishing Zn2+-dependent metaphase II arrest without Ca2+ release. Development (Cambridge, England) 137(16):2659–2669
Bernhardt ML, Kong BY, Kim AM, O'Halloran TV, Woodruff TK (2012) A zinc-dependent mechanism regulates meiotic progression in mammalian oocytes. Biol Reprod 86(4):114
Vidal F, Hidalgo J (2008) Effect of zinc and copper on preimplantation mouse embryo development in vitro and metallothionein levels. Zygote 1(3):225–229
Tamate K, Sengoku K, Ishikawa M (1995) The role of superoxide dismutase in the human ovary and fallopian tube. J Obstet Gynaecol (Tokyo, Japan) 21(4):401–409
Matsubayashi H, Kitaya K, Yamaguchi K, Nishiyama R, Takaya Y, Ishikawa T (2017) Is a high serum copper concentration a risk factor for implantation failure? BMC Res Notes 10(1):387
Guo C-H, Chen P-C, Yeh M-S, Hsiung D-Y, Wang C-L (2011) Cu/Zn ratios are associated with nutritional status, oxidative stress, inflammation, and immune abnormalities in patients on peritoneal dialysis. Clin Biochem 44(4):275–280
Rafeeinia A, Tabandeh A, Khajeniazi S, Marjani AJ (2014) Serum copper, zinc and lipid peroxidation in pregnant women with preeclampsia in gorgan. Open Biochem J 8:83
Cetin I, Berti C, Calabrese S (2010) Role of micronutrients in the periconceptional period. Hum Reprod Update 16(1):80–95
Li Y, Park J-S, Deng J-H, Bai Y (2006) Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J Bioenerg Biomembr 38(5-6):283–291
Guerin P, El Mouatassim S, Menezo Y (2001) Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update 7(2):175–189
Chen J, Zeng L, Xia T, Li S, Yan T, Wu S, Qiu G, Liu Z (2015) Toward a biomarker of oxidative stress: a fluorescent probe for exogenous and endogenous malondialdehyde in living cells. Anal Chem 87(16):8052–8056
Al-Gubory KH, Bolifraud P, Germain G, Nicole A, Ceballos-Picot I (2004) Antioxidant enzymatic defence systems in sheep corpus luteum throughout pregnancy. Reproduction (Cambridge, England) 128(6):767–774
Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ (2000) Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol 157(6):2111–2122
Qanungo S, Mukherjea M (2000) Ontogenic profile of some antioxidants and lipid peroxidation in human placental and fetal tissues. Mol Cell Biochem 215(1-2):11–19
Kim IH, Van Langendonckt A, Van Soom A, Vanroose G, Casi AL, Hendriksen PJ, Bevers MM (1999) Effect of exogenous glutathione on the in vitro fertilization of bovine oocytes. Theriogenology 52(3):537–547
Lubos E, Loscalzo J, Handy DE (2011) Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 15(7):1957–1997
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent was obtained from all the participants, and the Institutional Ethics Committee approved the study (approval number: 05-26.09.2018). The study was conducted in compliance with the Declaration of Helsinki.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tok, A., Özer, A., Baylan, F.A. et al. Copper/Zinc Ratio Can Be a Marker to Diagnose Ectopic Pregnancy and Is Associated with the Oxidative Stress Status of Ectopic Pregnancy Cases. Biol Trace Elem Res 199, 2096–2103 (2021). https://doi.org/10.1007/s12011-020-02327-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02327-0