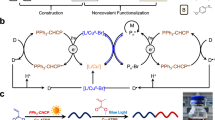
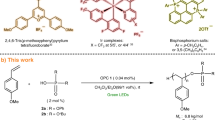
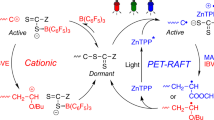
Abstract
Ring-opening polymerization of lactones has served as one of the most resourceful and versatile methods for the production of polyesters. However, the photocontrolled ring-opening polymerization of lactones with the advantages of being inexpensive, green, and noninvasive, is still rarely reported. In this work, we developed a series of composite photoacid generators containing a common photocatalyst and an onium salt to induce the living/controlled cationic ring-opening polymerization of lactones using benzyl alcohol or butyl alcohol as an initiator under visible light. The wavelength of the light could be easily adjustable by applying different photocatalysts. Moreover, radical species are generated concurrently during the electron transfer processes; as a result, simultaneous living/controlled cationic ring-opening polymerization of lactones and reversible addition–fragmentation chain transfer radical (RAFT) polymerization can be performed using hydroxy group capped trithiocarbonate to produce hybrid block copolymers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ikada Y, Tsuji H. Biodegradable polyesters for medical and ecological applications. Macromol Rapid Commun. 2000;21:117–32.
Tang X, Chen EYX. Toward Infinitely recyclable plastics derived from renewable cyclic esters. Chem. 2019;5:284–312.
Rainbolt EA, Washington KE, Biewer MC, Stefan MC. Recent developments in micellar drug carriers featuring substituted poly(ε-caprolactone)s. Polym Chem. 2015;6:2369–81.
Odian, G. Principles of polymerization. Hoboken, New Jersey: John Wiley & Sons; 2004.
Thomas CM. Stereocontrolled ring-opening polymerization of cyclic esters: synthesis of new polyester microstructures. Chem Soc Rev. 2010;39:165–73.
Yin Q, Yin L, Wang H, Cheng J. Synthesis and biomedical applications of functional poly (α-hydroxy acids) via ring-opening polymerization of O-carboxyanhydrides. Acc Chem Res. 2015;48:1777–87.
Bailey WJ, Gu J-M, Lin Y-N, Zheng Z-F, Zhou L-L. Ring‐opening polymerization of cyclic ketene acetals and unsaturated cyclic spiro ortho esters. Makromolekulare Chemie. Macromolecular Symposia. 1991;42–43:195–203. https://doi.org/10.1002/masy.19910420116.
Longo JM, Sanford MJ, Coates GW. Ring-opening copolymerization of epoxides and cyclic anhydrides with discrete metal complexes: structure–property relationships. Chem Rev. 2016;116:15167–97.
Hofman A, Słomkowski S, Penczek S. Structure of active centers and mechanism of the anionic polymerization of lactones. Die Makromol Chem. 1984;185:91–101.
Penczek S, Kubisa P, Matyjaszewski K. Cationic ring-opening polymerization of heterocyclic monomers. 1. Mechanisms. Advances in Polymer Science. 1980:37;1–144.
Nifant’ev I, Ivchenko P. Coordination ring-opening polymerization of cyclic esters: a critical overview of DFT modeling and visualization of the reaction mechanisms. Molecules. 2019;24:4117.
Sun X, Gao JP, Wang ZY. Bicyclic guanidinium tetraphenylborate: a photobase generator and a photocatalyst for living anionic ring-opening polymerization and cross-linking of polymeric materials containing ester and hydroxy groups. J Am Chem Soc. 2008;130:8130–1.
Coulembier O, Dove AP, Pratt RC, Sentman AC, Culkin DA, Mespouille L, et al. Latent, thermally activated organic catalysts for the on-demand living polymerization of lactide. Angew Chem Int Ed Engl. 2005;44:4964–8.
Qi M, Dong Q, Wang D, Byers JA. Electrochemically switchable ring-opening polymerization of lactide and cyclohexene oxide. J Am Chem Soc. 2018;140:5686–90.
Wei J, Diaconescu PL. Redox-switchable ring-opening polymerization with ferrocene derivatives. Acc Chem Res. 2019;52:415–24.
Osaki M, Takashima Y, Yamaguchi H, Harada A. Switching of polymerization activity of cinnamoyl-α-cyclodextrin. Org Biomol Chem. 2009;7:1646–51.
Neilson BM, Bielawski CW. Photoswitchable NHC-promoted ring-opening polymerizations. Chem Commun. 2013;49:5453–5.
Zivic N, Kuroishi PK, Dumur F, Gigmes D, Dove AP, Sardon H. Recent advances and challenges in the design of organic photoacid and photobase generators for polymerizations. Angew Chem Int Ed. 2019;58:10410–22.
Barker IA, Dove AP. Triarylsulfonium hexafluorophosphate salts as photoactivated acidic catalysts for ring-opening polymerisation. Chem Commun. 2013;49:1205–7.
Fu CK, Xu JT, Boyer C. Photoacid-mediated ring opening polymerization driven by visible light. Chem Commun. 2016;52:7126–9.
Yagci Y, Jockusch S, Turro NJ. Photoinitiated polymerization: advances, challenges, and opportunities. Macromolecules. 2010;43:6245–60.
Ciftci M, Yoshikawa Y, Yagci Y. Living cationic polymerization of vinyl ethers through a photoinduced radical oxidation/addition/deactivation sequence. Angew Chem Int Ed. 2017;56:519–23.
Dadashi-Silab S, Bildirir H, Dawson R, Thomas A, Yagci Y. Microporous thioxanthone polymers as heterogeneous photoinitiators for visible light induced free radical and cationic polymerizations. Macromolecules. 2014;47:4607–14.
Erdur S, Yilmaz G, Colak DG, Cianga I, Yagci Y. Poly(phenylenevinylene)s as sensitizers for visible light induced cationic polymerization. Macromolecules. 2014;47:7296–302.
Kahveci MU, Acik G, Yagci Y. Synthesis of block copolymers by combination of atom transfer radical polymerization and visible light-induced free radical promoted cationic polymerization. Macromol Rapid Commun. 2012;33:309–13.
Twilton J, Le C, Zhang P, Shaw MH, Evans RW, MacMillan DWC. The merger of transition metal and photocatalysis. Nat Rev Chem. 2017;1:1–19.
Prier CK, Rankic DA, MacMillan DWC. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem Rev. 2013;113:5322–63.
Narayanam JMR, Stephenson CRJ. Visible light photoredox catalysis: applications in organic synthesis. Chem Soc Rev. 2011;40:102–13.
Beatty JW, Stephenson CRJ. Amine functionalization via oxidative photoredox catalysis: methodology development and complex molecule synthesis. Acc Chem Res. 2015;48:1474–84.
Staveness D, Bosque I, Stephenson CRJ. Free radical chemistry enabled by visible light-induced electron transfer. Acc Chem Res. 2016;49:2295–306.
Rehm D, Weller A. Kinetics of fluorescence quenching by electron and H-atom transfer. Isr J Chem. 1970;8:259–71.
Ramette RW, Sandell EB. Rhodamine B equilibria. J Am Chem Soc. 1956;78:4872–8.
Pohlers G, Scaiano JC. A novel photometric method for the determination of photoacid generation efficiencies using benzothiazole and xanthene dyes as acid sensors. Chem Mater. 1997;9:3222–30.
Yagci YJM. Poly(phenylenevinylene)s as sensitizers for visible light induced cationic polymerization. Macromolecules. 47, 7296–302.
Okamoto Y. Cationic ring-opening polymerization of lactones in the presence of alcohol, Makromolekulare Chemie-Macromolecular Symposia. 1991:42-3;117–33.
Xu J, Shanmugam S, Duong HT, Boyer C. Organo-photocatalysts for photoinduced electron transfer-reversible addition–fragmentation chain transfer (PET-RAFT) polymerization. Polym Chem. 2015;6:5615–24.
Wu C, Chen H, Corrigan N, Jung K, Kan X, Li Z, et al. Computer-guided discovery of a pH-responsive organic photocatalyst and application for pH and light dual-gated polymerization. J Am Chem Soc. 2019;141:8207–20.
Shanmugam S, Xu J, Boyer C. Exploiting metalloporphyrins for selective living radical polymerization tunable over visible wavelengths. J Am Chem Soc. 2015;137:9174–85.
Xu J, Fu C, Shanmugam S, Hawker CJ, Moad G, Boyer C. Synthesis of discrete oligomers by sequential PET-RAFT single-unit monomer insertion. Angew Chem Int Ed. 2017;56:8376–83.
Riess G. Micellization of block copolymers. Prog Polym Sci. 2003;28:1107–70.
Cabral H, Miyata K, Osada K, Kataoka K. Block copolymer micelles in nanomedicine applications. Chem Rev. 2018;118:6844–92.
Feng HB, Lu XY, Wang WY, Kang N-G, Mays JW. Block copolymers: synthesis, self-assembly, and applications. Polymers. 2017;9:31.
Bates CM, Bates FS. 50th anniversary perspective: block polymers-pure potential. Macromolecules. 2017;50:3–22.
Flamigni L, Barbieri A, Sabatini C, Ventura B, Barigelletti F. Photochemistry and photophysics of coordination compounds: iridium. In: Balzani V, Campagna S, editors. Photochemistry and photophysics of coordination compounds II. Berlin, Heidelberg: Springer Berlin Heidelberg; 2007. pp. 143–203.
Grzeskowiak S, Narasimhan A, Rebeyev E, Joshi S, Brainard RL, Denbeaux G. Acid generation efficiency of EUV PAGs via low energy electron exposure. J Photopolym Sci Technol. 2016;29:453–8.
Podsiadły R, Podemska K, Szymczak AMJD. Novel visible photoinitiators systems for free-radical/cationic hybrid photopolymerization. Dyes and Pigments. 2011;91:422–6.
Jones G, Chatterjee SJTJoPC. Steric control of distance parameters and the yield of charge carriers in photochemical electron transfer. The quenching of eosin Y by hindered phenols. J Phys. Chem. 1988;92:6862–4.
Seely GR. The energetics of electron‐transfer reactions of chlorophyll and other compounds. Photochem Photobiol. 1978;27:639–54.
Acknowledgements
This work was financially supported by the National Natural Science Funds for Distinguished Young Scholars (51625305) and the National Natural Science Foundation of China (51273187, 21474097, and 21801234).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Xia, L., Zhang, Z. & You, YZ. Visible light-induced living/controlled cationic ring-opening polymerization of lactones. Polym J 52, 1323–1331 (2020). https://doi.org/10.1038/s41428-020-0394-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-020-0394-x