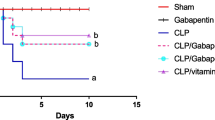
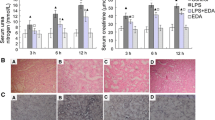
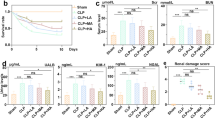
Abstract
Acute kidney injury (AKI) resulting from septic shock caused by sepsis is an important health problem encountered at rates of 55–73%. Increasing oxidative stress and inflammation following sepsis is a widely observed condition with rising mortality rates. The purpose of this study was to determine whether perindopril (PER) can prevent sepsis-associated AKI with its antioxidant, anti-inflammatory, and anti-apoptotic effects. The control group received an oral saline solution only for 4 days. Cecal ligation and puncture (CLP)–induced sepsis only was applied to the CLP group, while the CLP + PER (2 mg/kg) received CLP-induced sepsis together with 2 mg/kg PER via the oral route for 4 days before induction of sepsis. Finally, all rats were euthanized by anesthesia and sacrificed. TBARS, total SH levels and NF-κβ, TNF-α, and Caspase-3 expression were then calculated for statistical analysis. TBARS, total SH, NF-kβ/p65, TNF-a, and Caspase-3 levels increased in the CLP group. In contrast, oral administration of PER (2 mg/kg) to septic rats reduced TBARS levels and NF-kβ/p65, TNF-α, and Caspase-3 immunopositivity at biochemical analysis. PER treatment appears to be a promising method for preventing sepsis-induced acute kidney injury through its antioxidant anti-inflammation and anti-apoptotic activities.




Similar content being viewed by others
References
Mehta, Ravindra L., Josée Bouchard, Sharon B. Soroko, T. Alp Ikizler, Emil P. Paganini, Glenn M. Chertow, and Jonathan Himmelfarb. 2011. Sepsis as a cause and consequence of acute kidney injury: program to improve care in acute renal disease. Intensive Care Medicine 37: 241–248. https://doi.org/10.1007/s00134-010-2089-9.
Cecconi, Maurizio, Laura Evans, Mitchell Levy, and Andrew Rhodes. 2018. Sepsis and septic shock. The Lancet 392: 75–87. https://doi.org/10.1016/S0140-6736(18)30696-2.
Schrier, Robert W., and Wei Wang. 2004. Mechanisms of disease: Acute renal failure and sepsis. New England Journal of Medicine 351: 159–169 + 201. https://doi.org/10.1056/NEJMra032401.
Umbro, Ilaria, Giuseppe Gentile, Francesca Tinti, Paolo Muiesan, and Anna Paola Mitterhofer. 2016. Recent advances in pathophysiology and biomarkers of sepsis-induced acute kidney injury. Journal of Infection 72: 131–142. https://doi.org/10.1016/j.jinf.2015.11.008.
Jo, Sang Kyung, Su Ah. Sung, Won Yong Cho, Kang Jee Go, and Hyoung Kyu Kim. 2006. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrology, Dialysis, Transplantation 21: 1231–1239. https://doi.org/10.1093/ndt/gfk047.
Langenberg, C., L. Wan, M. Egi, C.N. May, and R. Bellomo. 2006. Renal blood flow in experimental septic acute renal failure. Kidney International 69: 1996–2002. https://doi.org/10.1038/sj.ki.5000440.
Li, Xing, Mu Genhua, Chunmei Song, Liangliang Zhou, Lei He, Qin Jin, and Lu. Zhongqian. 2018. Role of M2 macrophages in sepsis-induced acute kidney injury. Shock 50: 233–239. https://doi.org/10.1097/SHK.0000000000001006.
de Paulo Rodrigues, Francisco Adelvane, Alan Diego da Conceição Santos, Pedro Henrique Quintela Soares de Medeiros, Mara de Moura Gondim Prata, Tailane Caína de Souza Santos, James Almada da Silva, Gerly Anne de Castro Brito, et al. 2018. Gingerol suppresses sepsis-induced acute kidney injury by modulating methylsulfonylmethane and dimethylamine production. Scientific Reports 8: 1–10. https://doi.org/10.1038/s41598-018-30522-6.
Pavlakou, Paraskevi, Vassilios Liakopoulos, Theodoros Eleftheriadis, Michael Mitsis, and Evangelia Dounousi. 2017. Oxidative stress and acute kidney injury in critical illness: pathophysiologic mechanisms - biomarkers - interventions, and future perspectives. Oxidative Medicine and Cellular Longevity. https://doi.org/10.1155/2017/6193694.
Lin, Zhaoheng, Jing Jin, and Xiyun Shan. 2019. Fish oils protects against cecal ligation and puncture-induced septic acute kidney injury via the regulation of inflammation, oxidative stress and apoptosis. International Journal of Molecular Medicine 44: 1771–1780. https://doi.org/10.3892/ijmm.2019.4337.
Campos, Renata, Maria Heloísa Massola Shimizu, Rildo Aparecido Volpini, Ana Carolina de Bragança, Lucia Andrade, Fernanda Degobbi Tenório Quirino dos Santos Lopes, Clarice Olivo, Daniele Canale, and Antonio Carlos Seguro. 2012. N-acetylcysteine prevents pulmonary edema and acute kidney injury in rats with sepsis submitted to mechanical ventilation. American Journal of Physiology - Lung Cellular and Molecular Physiology 302: 640–650. https://doi.org/10.1152/ajplung.00097.2011.
Lushchak, Volodymyr I. 2012. Glutathione homeostasis and functions: potential targets for medical interventions. Journal of Amino Acids 2012: 1–26. https://doi.org/10.1155/2012/736837.
Rousta, Ali Mohammad, Seyed Mohamad Sadegh Mirahmadi, Alireza Shahmohammadi, Davood Nourabadi, Mohammad Reza Khajevand-Khazaei, Tourandokht Baluchnejadmojarad, and Mehrdad Roghani. 2018. Protective effect of sesamin in lipopolysaccharide-induced mouse model of acute kidney injury via attenuation of oxidative stress, inflammation, and apoptosis. Immunopharmacology and Immunotoxicology 40: 423–429. https://doi.org/10.1080/08923973.2018.1523926.
Cao, Yi Zhan, Yan Yang Tu, Xiang Chen, Bo Liang Wang, Yue Xia Zhong, and Ming Hua Liu. 2012. Protective effect of ulinastatin against murine models of sepsis: inhibition of TNF-α and IL-6 and augmentation of IL-10 and IL-13. Experimental and Toxicologic Pathology 64: 543–547. https://doi.org/10.1016/j.etp.2010.11.011.
Gan, Y., S. Tao, D. Cao, H. Xie, and Q. Zeng. 2017. Protection of resveratrol on acute kidney injury in septic rats. Human and Experimental Toxicology 36: 1015–1022. https://doi.org/10.1177/0960327116678298.
Luo, Cong-Juan, Feng Luo, Bu Quan-Dong, Wei Jiang, Wei Zhang, Xue-Mei Liu, Che Lin, et al. 2019. Protective effects of resveratrol on acute kidney injury in rats with sepsis. Biomedical Papers. 164: 49–56. https://doi.org/10.5507/bp.2019.006.
Wang, Nian, Li Mao, Liu Yang, Jiang Zou, Ke Liu, Meidong Liu, Huali Zhang, Xianzhong Xiao, and Kangkai Wang. 2017. Resveratrol protects against early polymicrobial sepsis-induced acute kidney injury through inhibiting endoplasmic reticulum stress-activated NF-κB pathway. Oncotarget 8: 36449–36461. https://doi.org/10.18632/oncotarget.16860.
Sun, Guodong, Wei Yang, Zhang Yang, and Mingyan Zhao. 2017. Esculentoside A ameliorates cecal ligation and puncture-induced acute kidney injury in rats. Experimental Animals 66: 303–312. https://doi.org/10.1538/expanim.16-0102.
Li, Guofu, Linlin Gao, Jia Jia, Xiaoying Gong, Bin Zang, and Weimin Chen. 2014. α-Lipoic acid prolongs survival and attenuates acute kidney injury in a rat model of sepsis. Clinical and Experimental Pharmacology and Physiology 41: 459–468. https://doi.org/10.1111/1440-1681.12244.
Gao, Li, Wei Feng Wu, Lei Dong, Gui Ling Ren, Hai Di Li, Qin Yang, Xiao Feng Li, et al. 2016. Protocatechuic aldehyde attenuates cisplatin-induced acute kidney injury by suppressing nox-mediated oxidative stress and renal inflammation. Frontiers in Pharmacology 7: 1–16. https://doi.org/10.3389/fphar.2016.00479.
Shum, Hoi Ping, Wing Wa Yan, and Tak Mao Chan. 2016. Recent knowledge on the pathophysiology of septic acute kidney injury: a narrative review. Journal of Critical Care 31: 82–89. https://doi.org/10.1016/j.jcrc.2015.09.017.
Kostakoglu, Ugur, Atilla Topcu, Mehtap Atak, Levent Tumkaya, Tolga Mercantepe, and Huseyin Avni Uydu. 2020. The protective effects of angiotensin-converting enzyme inhibitor against cecal ligation and puncture-induced sepsis via oxidative stress and inflammation. Life Sciences 241: 117051. https://doi.org/10.1016/j.lfs.2019.117051.
Ali, Mohammed Ragab Abdel Aziz, Amira Morad Hussein Abo-Youssef, Basim Anwar Shehata Messiha, and Mahmoud Mohamed Khattab. 2016. Tempol and perindopril protect against lipopolysaccharide-induced cognition impairment and amyloidogenesis by modulating brain-derived neurotropic factor, neuroinflammation and oxido-nitrosative stress. Naunyn-Schmiedeberg’s Archives of Pharmacology 389: 637–656. https://doi.org/10.1007/s00210-016-1234-6.
Ancion, Arnaud, Julien Tridetti, Mai-Linh Nguyen Trung, Cécile Oury, and Patrizio Lancellotti. 2019. A review of the role of bradykinin and nitric oxide in the cardioprotective action of angiotensin-converting enzyme inhibitors: focus on perindopril. Cardiology and Therapy 8: 179–191. https://doi.org/10.1007/s40119-019-00150-w.
Shalkami, Abdel Gawad S., Mohamed I.A. Hassan, and Ahmed A. Abd El-Ghany. 2018. Perindopril regulates the inflammatory mediators, NF-κB/TNF-α/IL-6, and apoptosis in cisplatin-induced renal dysfunction. Naunyn-Schmiedeberg’s Archives of Pharmacology 391: 1247–1255. https://doi.org/10.1007/s00210-018-1550-0.
Tang, S.C.W., J.C.K. Leung, L.Y.Y. Chan, A.A. Eddy, and K.N. Lai. 2008. Angiotensin converting enzyme inhibitor but not angiotensin receptor blockade or statin ameliorates murine adriamycin nephropathy. Kidney International 73: 288–299. https://doi.org/10.1038/sj.ki.5002674.
Mashhoody, Tahereh, Karim Rastegar, and Fatemeh Zal. 2014. Perindopril may improve the hippocampal reduced glutathione content in rats. Advanced Pharmaceutical Bulletin 4: 155–159. https://doi.org/10.5681/apb.2014.023.
Rittirsch, Daniel, Markus S. Huber-lang, Michael A. Flierl, and Peter A. Ward. 2009. Immunodesing of experimental sepsis by cecal ligation and puncture. Nature Protocols 4: 31–36. https://doi.org/10.1038/nprot.2008.214.Immunodesign.
Cinar, Irfan, Busra Sirin, Pelin Aydin, Erdem Toktay, Elif Cadirci, Iclal Halici, and Zekai Halici. 2019. Ameliorative effect of gossypin against acute lung injury in experimental sepsis model of rats. Life Sciences 221: 327–334. https://doi.org/10.1016/j.lfs.2019.02.039.
Rojas, Denise Bertin, Tanise Gemelli, Rodrigo Binkowski De Andrade, Aline Guimarães Campos, Carlos Severo Dutra-Filho, and Clóvis Milton Duval Wannmacher. 2012. Administration of histidine to female rats induces changes in oxidative status in cortex and hippocampus of the offspring. Neurochemical Research 37: 1031–1036. https://doi.org/10.1007/s11064-012-0703-7.
Ohkawa, Hiroshi, Nobuko Ohishi, and Kunio Yagi. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry 95: 351–358. https://doi.org/10.1016/0003-2697(79)90738-3.
Başol, Nurşah, Oytun Erbaş, Türker Çavuşoğlu, Ayfer Meral, and Utku Ateş. 2016. Sepsis nedenli akut böbrek hasarında agomelatinin etkilerinin değerlendirilmesi. Ulusal Travma ve Acil Cerrahi Dergisi 22: 121–126. https://doi.org/10.5505/tjtes.2015.29499.
Peña-Bautista, Carmen, Miguel Baquero, Máximo Vento, and Consuelo Cháfer-Pericás. 2019. Free radicals in Alzheimer’s disease: lipid peroxidation biomarkers. Clinica Chimica Acta 491: 85–90. https://doi.org/10.1016/j.cca.2019.01.021.
Wheeler, Derek S. 2013. Oxidative stress in critically ill children with sepsis. Open Inflammation Journal 4: 74–81. https://doi.org/10.2174/1875041901104010074.Oxidative.
Zhang, Zhijie, Huatang Zhao, Dongjian Ge, Shanshan Wang, and Bin Qi. 2019. β-casomorphin-7 ameliorates sepsis-induced acute kidney injury by targeting NF-κB pathway. Medical Science Monitor 25: 121–127. https://doi.org/10.12659/MSM.912730.
Chuang, C.H., C.K. Yang, P.H. Wu, Y. Zhang, and P.J. Yang. 2018. Acute renal injury induced by endotoxic shock in rats is alleviated via PI3K/Nrf2 pathway. European Review for Medical and Pharmacological Sciences 22: 5394–5401. https://doi.org/10.26355/eurrev_201808_15742.
Bar-Or, David, Raphael Bar-Or, Leonard T. Rael, and Edward N. Brody. 2015. Oxidative stress in severe acute illness. Redox Biology 4: 340–345. https://doi.org/10.1016/j.redox.2015.01.006.
Lim, Jinhwan, Samiha Ali, Lisa S. Liao, Emily S. Nguyen, Laura Ortiz, Samantha Reshel, and Ulrike Luderer. 2020. Antioxidant supplementation partially rescues accelerated ovarian follicle loss, but not oocyte quality, of glutathione deficient mice. Biology of Reproduction: 1–45. https://doi.org/10.1093/biolre/ioaa009.
Chen, Guang Dao, Jun Liang Zhang, Yi Ting Chen, Ju Xing Zhang, Tao Wang, and Qi Yi Zeng. 2018. Insulin alleviates mitochondrial oxidative stress involving upregulation of superoxide dismutase 2 and uncoupling protein 2 in septic acute kidney injury. Experimental and Therapeutic Medicine 15: 3967–3975. https://doi.org/10.3892/etm.2018.5890.
Xia, Shilin, Hongli Lin, Han Liu, Zhidan Lu, Hui Wang, Songtao Fan, and Nan Li. 2019. Honokiol attenuates sepsis-associated acute kidney injury via the inhibition of oxidative stress and inflammation. Inflammation 42: 826–834. https://doi.org/10.1007/s10753-018-0937-x.
Yan, Xi Xiang, Ai Dong Zheng, Zhen En Zhang, Guo Cui Pan, and Wen Zhou. 2019. Protective effect of pantoprazole against sepsis-induced acute lung and kidney injury in rats. American Journal of Translational Research 11: 5197–5211.
Knight, Jasper, Chris Caseldine, and Maxwell T. Boykoff. 2010. Forum review: forum review. Geographical Journal 176: 267–269. https://doi.org/10.1111/j.1475-4959.2010.00371.x.
Qin, Yi, Guizhen Wang, and Zhiyong Peng. 2019. MicroRNA-191-5p diminished sepsis-induced acute kidney injury through targeting oxidative stress responsive 1 in rat models. Bioscience Reports 39: 1–11. https://doi.org/10.1042/BSR20190548.
Zhao, Yuan, Xiujing Feng, Bei Li, Jichen Sha, Chaoran Wang, Tianyuan Yang, Hailin Cui, and Honggang Fan. 2020. Dexmedetomidine protects against lipopolysaccharide-induced acute kidney injury by enhancing autophagy through inhibition of the PI3K/AKT/mTOR pathway. Frontiers in Pharmacology 11: 1–13. https://doi.org/10.3389/fphar.2020.00128.
Liu, Lin, Yijin Song, Ming Zhao, Zhuwen Yi, and Qiyi Zeng. 2015. Protective effects of edaravone, a free radical scavenger, on lipopolysaccharide-induced acute kidney injury in a rat model of sepsis. International Urology and Nephrology 47. 1745–1752. https://doi.org/10.1007/s11255-015-1070-5.
Zhu, Zhenyu, Huihui Li, Wanli Chen, Yameng Cui, Anan Huang, and Xin Qi. 2020. Perindopril improves cardiac function by enhancing the expression of SIRT3 and PGC-1α in a rat model of isoproterenol-induced cardiomyopathy. Frontiers in Pharmacology 11: 1–11. https://doi.org/10.3389/fphar.2020.00094.
Author information
Authors and Affiliations
Contributions
UK, TM, and SB conceived and designed the research. UK and LT conducted the experiments. HKY, HAU, and TM contributed new reagents or analytical tools. UK, HAU, HKY, TM, and SB analyzed the data. UK and TM wrote the manuscript. All authors read and approved the final text.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study protocol was approved by the Recep Tayyip Erdogan University animal care committee (approval number. 2020/05 dated 28.02.2020).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kostakoglu, U., Mercantepe, T., Yilmaz, H.K. et al. The Protective Effects of Perindopril Against Acute Kidney Damage Caused by Septic Shock. Inflammation 44, 148–159 (2021). https://doi.org/10.1007/s10753-020-01316-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01316-8