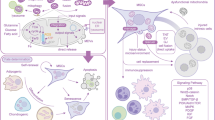
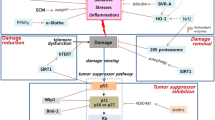
Abstract
Mesenchymal stem cells (MSCs) hold enormous potential for the treatment of immune-related conditions and degenerative diseases, owing to their self-renewal and multilineage differentiation capabilities. Nevertheless, cellular senescence significantly impacts the quantity and quality of MSCs, limiting their clinical use. Mitochondria play essential roles in energy production by oxidative phosphorylation and metabolism of energy sources via the tricarboxylic acid cycle. Therefore, mitochondrial dysfunction is a primary cause of senescence in MSCs. Herein, we summarize the current knowledge regarding the mechanisms underlying mitochondrial dysfunction–associated cellular senescence. We also discuss potential methods to prevent or even reverse MSC senescence.

Similar content being viewed by others
References
Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, Longerich T, Sansom OJ, Benitah SA, Zender L, Gil J (2013) A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15:978–990
Alessio N, Riccitiello F, Squillaro T, Capasso S, Del Gaudio S, Di Bernardo G, Cipollaro M, Melone MAB, Peluso G, Galderisi U (2018) Neural stem cells from a mouse model of Rett syndrome are prone to senescence, show reduced capacity to cope with genotoxic stress, and are impaired in the differentiation process. Exp Mol Med 50:1–1
Beaupere C, Garcia M, Larghero J, Fève B, Capeau J, Lagathu C (2015) The HIV proteins Tat and Nef promote human bone marrow mesenchymal stem cell senescence and alter osteoblastic differentiation. Aging Cell 14:534–546
Böttinger L, Guiard B, Oeljeklaus S, Kulawiak B, Zufall N, Wiedemann N, Warscheid B, van der Laan M, Becker T (2013) A complex of Cox4 and mitochondrial Hsp70 plays an important role in the assembly of the cytochrome c oxidase. Mol Biol Cell 24:2609–2619
Brzezinski A (1997) Melatonin in humans. N Engl J Med 336:186–195
Capaldi RA (1990) Structure and function of cytochrome c oxidase. Annu Rev Biochem 59:569–596
Chapman J, Fielder E, Passos JF (2019) Mitochondrial dysfunction and cell senescence: deciphering a complex relationship. FEBS Lett 593:1566–1579
Checler F, Goiran T, Alves da Costa C (2018) Nuclear TP53: an unraveled function as transcriptional repressor of PINK1. Autophagy 14:1099–1101
Chichester L, Wylie AT, Craft S, Kavanagh K (2015) Muscle heat shock protein 70 predicts insulin resistance with aging. J Gerontol A Biol Sci Med Sci 70:155–162
Drew BG, Ribas V, Le JA, Henstridge DC, Phun J, Zhou Z, Soleymani T, Daraei P, Sitz D, Vergnes L, Wanagat J, Reue K, Febbraio MA, Hevener AL (2014) HSP72 is a mitochondrial stress sensor critical for Parkin action, oxidative metabolism, and insulin sensitivity in skeletal muscle. Diabetes 63:1488–1505
Fan P, Yu XY, Xie XH, Chen CH, Zhang P, Yang C, Peng X, Wang YT (2019) Mitophagy is a protective response against oxidative damage in bone marrow mesenchymal stem cells. Life Sci 229:36–45. https://doi.org/10.1016/j.lfs.2019.05.027
Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, Lautrup S, Hasan-Olive MM, Caponio D, Dan X, Rocktäschel P, Croteau DL, Akbari M, Greig NH, Fladby T, Nilsen H, Cader MZ, Mattson MP, Tavernarakis N, Bohr VA (2019) Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci 22:401–412
Friedenstein AJ, Gorskaja JF, Kulagina NN (1976) Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol 4:267–274
Fujiwara M, Tian L, Le PT, DeMambro VE, Becker KA, Rosen CJ, Guntur AR (2019) The mitophagy receptor Bcl-2-like protein 13 stimulates adipogenesis by regulating mitochondrial oxidative phosphorylation and apoptosis in mice. J Biol Chem 294:12683–12694. https://doi.org/10.1074/jbc.RA119.008630
García-Prat L, Martínez-Vicente M, Perdiguero E, Ortet L, Rodríguez-Ubreva J, Rebollo E, Ruiz-Bonilla V, Gutarra S, Ballestar E, Serrano AL, Sandri M, Muñoz-Cánoves P (2016) Autophagy maintains stemness by preventing senescence. Nature 529:37–42
Geissler S, Textor M, Kühnisch J, Könnig D, Klein O, Ode A, Pfitzner T, Adjaye J, Kasper G, Duda GN (2012) Functional comparison of chronological and in vitro aging: differential role of the cytoskeleton and mitochondria in mesenchymal stromal cells. PLoS One 7:e52700–e52700
Hüttemann M, Kadenbach B, Grossman LI (2001) Mammalian subunit IV isoforms of cytochrome c oxidase. Gene 267:111–123
Ito S, Araya J, Kurita Y, Kobayashi K, Takasaka N, Yoshida M, Hara H, Minagawa S, Wakui H, Fujii S, Kojima J, Shimizu K, Numata T, Kawaishi M, Odaka M, Morikawa T, Harada T, Nishimura SL, Kaneko Y, Nakayama K, Kuwano K (2015) PARK2-mediated mitophagy is involved in regulation of HBEC senescence in COPD pathogenesis. Autophagy 11:547–559
Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N, Strouboulis J, Wolffe AP (1998) Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet 19:187–191
Kim J, Piao Y, Pak YK, Chung D, Han YM, Hong JS, Jun EJ, Shim J-Y, Choi J, Kim CJ (2015) Umbilical cord mesenchymal stromal cells affected by gestational diabetes mellitus display premature aging and mitochondrial dysfunction. Stem Cells Dev 24:575–586
Kornicka K, Szłapka-Kosarzewska J, Śmieszek A, Marycz K (2019) 5-Azacytydine and resveratrol reverse senescence and ageing of adipose stem cells via modulation of mitochondrial dynamics and autophagy. J Cell Mol Med 23:237–259
Korolchuk VI, Miwa S, Carroll B, von Zglinicki T (2017) Mitochondria in cell senescence: is mitophagy the weakest link? EBioMedicine 21:7–13
Kurpinski K, Jang D-J, Bhattacharya S, Rydberg B, Chu J, So J, Wyrobek A, Li S, Wang D (2009) Differential effects of x-rays and high-energy 56Fe ions on human mesenchymal stem cells. Int J Radiat Oncol Biol Phys 73:869–877
Lee JH, Yoon YM, Song K-H, Noh H, Lee SH (2020) Melatonin suppresses senescence-derived mitochondrial dysfunction in mesenchymal stem cells via the HSPA1L-mitophagy pathway. Aging Cell:e13111-e13111
Li Y, Wu Q, Wang Y, Li L, Bu H, Bao J (2017) Senescence of mesenchymal stem cells (review). Int J Mol Med 39:775–782
Martellucci S, Santacroce C, Santilli F, Piccoli L, Delle Monache S, Angelucci A, Misasi R, Sorice M, Mattei V (2019) Cellular and molecular mechanisms mediated by recPrP(C) involved in the neuronal differentiation process of mesenchymal stem cells. Int J Mol Sci 20:345
Matsuda N, Tanaka K (2010) Uncovering the roles of PINK1 and Parkin in mitophagy. Autophagy 6:952–954
Nelson G, Kucheryavenko O, Wordsworth J, von Zglinicki T (2018) The senescent bystander effect is caused by ROS-activated NF-κB signalling. Mech Ageing Dev 170:30–36
Ntege EH, Sunami H, Shimizu Y (2020) Advances in regenerative therapy: a review of the literature and future directions. Regen Ther 14:136–153
Onodera Y, Teramura T, Takehara T, Obora K, Mori T, Fukuda K (2017) miR-155 induces ROS generation through downregulation of antioxidation-related genes in mesenchymal stem cells. Aging Cell 16:1369–1380
Passos JF, Saretzki G, Ahmed S, Nelson G, Richter T, Peters H, Wappler I, Birket MJ, Harold G, Schaeuble K, Birch-Machin MA, Kirkwood TBL, von Zglinicki T (2007) Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol 5:e110–e110
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Schofield JH, Schafer ZT (2020) Mitochondrial ROS and mitophagy: a complex and nuanced relationship. Antioxid Redox Signal. https://doi.org/10.1089/ars.2020.8058
Sen R, Baltimore D (1986) Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell 47:921–928
Seok, J., Jung, H.S., Park, S., Lee, J.O., Kim, C.J., Kim, G.J. (2020) Alteration of fatty acid oxidation by increased CPT1A on replicative senescence of placenta-derived mesenchymal stem cells. Stem Cell Res Ther 11, 1–1
Squillaro T, Alessio N, Capasso S, Di Bernardo G, Melone MAB, Peluso G, Galderisi U (2019) Senescence phenomena and metabolic alteration in mesenchymal stromal cells from a mouse model of Rett syndrome. Int J Mol Sci 20:2508
Stab BR, Martinez L, Grismaldo A, Lerma A, Gutiérrez ML, Barrera LA, Sutachan JJ, Albarracín SL (2016) Mitochondrial functional changes characterization in young and senescent human adipose derived MSCs. Front Aging Neurosci 8:1–10. https://doi.org/10.3389/fnagi.2016.00299
Tsujimoto T, Mori T, Houri K, Onodera Y, Takehara T, Shigi K, Nakao S, Teramura T, Fukuda K (2020) miR-155 inhibits mitophagy through suppression of BAG5, a partner protein of PINK1. Biochem Biophys Res Commun 523:707–712
Turinetto V, Vitale E, Giachino C (2016) Senescence in human mesenchymal stem cells: functional changes and implications in stem cell-based therapy. Int J Mol Sci 17:1164
Wang D, Jang D-J (2009) Protein kinase CK2 regulates cytoskeletal reorganization during ionizing radiation-induced senescence of human mesenchymal stem cells. Cancer Res 69:8200–8207
Wang Y, Han Z-B, Song Y-P, Han ZC (2012) Safety of mesenchymal stem cells for clinical application. Stem Cells Int 2012:652034–652034
Wang L, Wang J, Tang Y, Shen H-M (2018) PTEN-L puts a brake on mitophagy. Autophagy 14:2023–2025
Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, Shirakawa K, Lim HW, Davis SS, Ramanathan A, Gerencser AA, Verdin E, Campisi J (2016) Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab 23:303–314
Yang F, Yan G, Li Y, Han Z, Zhang L, Chen S, Feng C, Huang Q, Ding F, Yu Y, Bi C, Cai B, Yang L (2016) Astragalus polysaccharide attenuated iron overload-induced dysfunction of mesenchymal stem cells via suppressing mitochondrial ROS. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology 39:1369–1379
Yin F, Yan J, Zhao Y, Guo KJ, Zhang ZL, Li AP, Meng CY, Guo L (2019) Bone marrow mesenchymal stem cells repair Cr (VI)- injured kidney by regulating mitochondria-mediated apoptosis and mitophagy mediated via the MAPK signaling pathway. Ecotoxicol Environ Saf 176:234–241. https://doi.org/10.1016/j.ecoenv.2019.03.093
Yoon YM, Kim S, Han Y-S, Yun CW, Lee JH, Noh H, Lee SH (2019) TUDCA-treated chronic kidney disease-derived hMSCs improve therapeutic efficacy in ischemic disease via PrP(C). Redox Biol 22:101144–101144
Yoon YM, Lee JH, Song K-H, Noh H, Lee SH (2020) Melatonin-stimulated exosomes enhance the regenerative potential of chronic kidney disease-derived mesenchymal stem/stromal cells via cellular prion proteins. J Pineal Res:e12632-e12632
Yu D, Du Z, Pian L, Li T, Wen X, Li W, Kim S-J, Xiao J, Cohen P, Cui J, Hoffman AR, Hu J-F (2017) Mitochondrial DNA hypomethylation is a biomarker associated with induced senescence in human fetal heart mesenchymal stem cells. Stem Cells Int 2017:1764549–1764549
Zhang F, Peng W, Zhang J, Dong W, Wu J, Wang T, Xie Z (2020) P53 and Parkin co-regulate mitophagy in bone marrow mesenchymal stem cells to promote the repair of early steroid-induced osteonecrosis of the femoral head. Cell Death Dis 11:42–42
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 81874007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Y., Liu, Y., Chen, E. et al. The role of mitochondrial dysfunction in mesenchymal stem cell senescence. Cell Tissue Res 382, 457–462 (2020). https://doi.org/10.1007/s00441-020-03272-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-020-03272-z