Highlights
-
Current progress in preparations, structures, and physicochemical properties of two-dimensional photo-catalyst materials and environmental remediation.
-
Propose approaches of diverse of two-dimensional photo-catalyst materials-based nanoplatforms, optimization strategies to enhance activity, and their diverse applications.
-
Current challenges and potential advancement of the emerging of two-dimensional photo-catalyst materials.
Abstract
The successful photo-catalyst library gives significant information on feature that affects photo-catalytic performance and proposes new materials. Competency is considerably significant to form multi-functional photo-catalysts with flexible characteristics. Since recently, two-dimensional materials (2DMs) gained much attention from researchers, due to their unique thickness-dependent uses, mainly for photo-catalytic, outstanding chemical and physical properties. Photo-catalytic water splitting and hydrogen (H2) evolution by plentiful compounds as electron (e−) donors is estimated to participate in constructing clean method for solar H2-formation. Heterogeneous photo-catalysis received much research attention caused by their applications to tackle numerous energy and environmental issues. This broad review explains progress regarding 2DMs, significance in structure, and catalytic results. We will discuss in detail current progresses of approaches for adjusting 2DMs-based photo-catalysts to assess their photo-activity including doping, hetero-structure scheme, and functional formation assembly. Suggested plans, e.g., doping and sensitization of semiconducting 2DMs, increasing electrical conductance, improving catalytic active sites, strengthening interface coupling in semiconductors (SCs) 2DMs, forming nano-structures, building multi-junction nano-composites, increasing photo-stability of SCs, and using combined results of adapted approaches, are summed up. Hence, to further improve 2DMs photo-catalyst properties, hetero-structure design-based 2DMs’ photo-catalyst basic mechanism is also reviewed.

Similar content being viewed by others
1 Introduction
The sustainable energy and chemical supplies demands are very essential for modern society that is necessary for our transportation, prosperity, and daily simplicity. The world’s almost 85% energy demands are fulfilled by fossil fuels-based energy production. Therefore, an increasing progress in modern society for pollution-free energy production gains attention of the researchers in all fields. In the past, larger part of worldwide energy formation was based on fossil fuel, which increases environment pollution and hence causes global warming [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. In this modern society, development in different industries causes rapid population growth, which is further estimated to increase by two factors: the current energy required by 2050 to run industries around globe and their household uses [2,3,4, 15,16,17,18,19,20,21,22,23,24,25,26]. Presently, world’s energy supplies are mainly reliant on fossil fuels, for example, coal, petroleum, and natural gases, which are quickly being spent. Utilization of fossil fuels will certainly cause particular gases’ emission which are very injurious to the environment. Consequently, innovative findings in science and engineering are proceeded to address the barriers for efficient energy formation and environmental safety. Hence, production of the sustainable/renewable energy is a solution to meet up the rising worldwide energy demand and especially to solve environmental pollution issues [2,3,4, 27]. Conversely, fossil fuel-based energy is also widely used in chemical production on industrial level via inorganic/organic transformations by applying high-temperature/pressure circumstances. Although fossil fuel supplies are possibly sufficient for some next generations, durable cost of fossil fuels is undesirable because of un-sustainability of fossil fuels recognized as partial assets and rising greenhouse gases certified to enormous release of CO2-like hazard gases. The technical challenges to increase an industrially talented chemical process to protect a clean, renewable energy and to reduce harmful ecological impact are connected with the use of fossil fuels [2, 3, 19, 22]. In this regard, renewable sustainable energy production is one of the significant solutions, especially hydrogen (H2)-based energy creation by photo-catalysts as well as electrocatalysts [2,3,4, 18, 19, 21, 25]. Here, we will mainly concern on photo-catalysts. The H2 has the maximum energy contented per weight in combustion fuels and manufactures simply water (H2O) as by-product [3, 4, 19]. Thus far, straightforward transfer of solar energy to fuel energy (H2) and chemical energy was viewed as one of the green renewable ways to deal with energy and environmental pollution issues in the future [2,3,4, 17, 18, 21, 23,24,25]. Hence, H2 is considered as an ultra-clean, powerful, environment friendly, and hopeful another choice for meeting the future fuel necessities with environmental safety by less release of greenhouse gasses [2,3,4, 17,18,19, 21, 23, 24].
2 Basic Properties of Photo-catalysis
2.1 Merits of Photo-catalysis
The considerable reliance of worldwide economy on non-renewable and geopolitical susceptible fossil fuel energies has led to necessity in advance technologies to protect alternative clean and renewable energy supplies. In between different renewable energy sources (i.e., wind, tidal, hydroelectric, ocean currents, biomass, geothermal, and solar), solar energy is by far the most abundant, low cost, pollution free, and sustainable. Even though the total solar energy the earth receives for one hour is greater compared to annual global energy expenditure, the most serious challenge remains collection and storage of this very diffuse form of energy to facilitate real-world application and non-interrupted fuel supply. Photo-catalysis can be basically explained as a method wherein photo-generated electrons (e−s) and holes (h+s) induce targeted redox reactions on light absorbers and/or co-catalysts loaded on it. A range of other invented renewable energy schemes, semiconductor (SC)-based photo-catalysis, in which infinite and clean solar energy can be acquired as a possible technology [28] achieved great interdisciplinary concentration for their various probabilities in energy and environmental uses. Efficient transformation of solar energy to solar fuel using photo-catalytic method was measured as very eventual enduring maneuver to resolve global energy and environmental concerns [29]. Naturally abundant sunlight and H2O splitting-based production of H2 by using sunlight were verified as regenerative, environment friendly, and vast techniques to resolve energy disaster and environmental pollution. In photo-catalysis method, a steady and capable photo-catalyst is an important aspect to attain a high efficiency of H2. For energy crises as well as environmental issues, SC-based photo-catalysis has enormous ability to guarantee long-lasting and sustainable development, because of direct consumption of green solar energy for formation of important H2 fuels and degradation of organic pollutants. Generally, four steps take place in the photo-catalytic process:
-
1.
Light absorption
-
2.
Creation of photo-generated (e−–h+)-pairs
-
3.
Movement and recombination of photo-generated (e−–h+)
-
4.
Redox reactions at photo-catalysts surface
How to understand it proficiently is very demanding, both kinetically and thermodynamically. The complexities lie in the subsequent features:
-
1.
Maximum yielding of solar energy (mainly visible (vis) light) to produce enough energetic e−s/h+s
-
2.
High mobility and long dispersion length of photo-generated e−s/h+s to suppress bulk recombination
-
3.
Sufficiently strong reduction power of photo-generated e−s and h+s to persuade reactions, specially H2O oxidation that demands four e−s
-
4.
Plentiful surface locations for forward target reactions as an alternative of back reactions (e.g., H2 and O2 reaction to fabricate H2O)
Such four subjects represent a significant research pathway. Moreover, three steps span a huge timescale from 10−15 to 10−1 s. What’s more demanding are intrinsic conflicts between necessities for three key steps. Minimum three factors are considered here:
-
1.
Increasing light absorption range (reduced bandgaps) generally leads to small reduction capability of photo-generated e-s and/or a lower oxidation capability of photo-generated h+s;
-
2.
The very low mobility of h+s compared to e-s in most SCs does not support rate-determining H2O oxidation reactions,
-
3.
The difference in random distributions of oxidation and reduction reaction sites and required migration of e−s/h+s in diverse directions.
The strong underlying conflicts connected with photo-physical process, electronic properties, and catalysis principles build recognition of highly efficient photo-catalysis as a very challenging process. To solve these challenges, it is significantly important to accurately control every fundamental step depending on a comprehensive consideration of photo-catalysis and structure property interactions. Therefore, first we are going to explain SC materials’ suitability for photo-catalytic nature.
2.2 General Selection of SC Photo-catalytic Materials
Generally, the photo-catalysis is an accelerated photo-reaction method in existence of SC photo-catalyst, in which photons with energy hv ≥ Eg (Eg = band gap (BG) energy) of photo-catalyst are adsorbed to photoexcite free electrons (e−s) to conduction band (CB), creating holes (h+s) in valence band (VB). Photo-generated (e−–h+)-pairs participate in an important part for solar energy transfer method, for example, solar H2O splitting, CO2 reduction, and photo-catalytic pollutant degradation. Although photo-generated carriers in excited states are less stable, they recombine easily, which results in low conversion effectiveness of photo-catalysis [30]. By the way, since discovery of photo-catalytic H2O splitting with TiO2 in 1972, great effort was applied in progress of photo-catalysts for an efficient photo-catalytic method [31]. The SC-based photo-catalysis concerned huge research attention, [32] since it was considered very novel solution to manage energy deficiency and environmental pollution problems [33]. The sunlight as an exterior driving force can split H2O into H2 and O2, reduce CO2 to chemical and valuable fuel, as well as terminate pollutants entirely [34]. Normally, the main significant efforts in the photo-catalytic development are categorized as light absorption, charge separation, transfer, and surface redox responses. By irradiation of photo-catalysts, it absorbed sunlight, which excites to produce (e−–h+)-pairs, when hv ≥ Eg, leaving e-s in CB and h+s in VB, respectively. After that, photo-generated e–s and h+s are diffused to material surface and also transferred to surface active sites, prior to connection with surface reactions. Sometimes, charge carrier’s recombination occurs and crystal structure, particle size, crystallinity, surface morphology, etc., strongly influenced separation efficiency. At last, target molecules are adsorbed on the surface of materials and experience charge addition development and desorption to make final results [35].
In between (e−–h+)-recombination process, unnecessary heat is created, which causes a negative role in photo-catalytic production. In the photo-catalysis method, a stable and efficient photo-catalyst is an imperative feature to get high efficiency of H2. Additionally, the driving force of solar light photo-catalysis demands suitable SCs to perform various photo-catalytic responses, for example, H2O splitting to manufacture O2 and H2, CO2 reduction to hydrocarbon fuels, degradation of organic pollutants, disinfection of bacteria, and selective formation of organic compounds [36]. The milestone occurrence of photo-catalytic H2O splitting, by TiO2-based electrodes in an ultraviolet (UV) light, was started from revolutionary research co-authored by Fujishima and Honda [37]. In 1976, photo-catalytic degradation of organic contaminants was studied by Carey et al. [38] using TiO2 in aqueous suspension. In 1979, Inoue and co-authors examined photo-catalytic reduction of CO2 to a range of organic compounds by SC materials, for example, TiO2, SiC, ZnO, CdS, and GaP, in aqueous solution. After that, various considerable progresses were made in the formation of very proficient SC-based photo-catalysts. Up to now, several SC photo-catalysts were exploited and utilized in H2O splitting. Based on composition, photo-catalysts are usually classified into three kinds:
-
1.
Metal oxides (MOs)
-
2.
Metal chalcogenides
-
3.
Metal-free photo-catalysts
So far, hundreds of SC materials are discovered for different photo-catalytic uses by tuning a range from composition, electronic, and crystal structure. While important accomplishment was achieved in optimizing photo-catalytic performance, most photo-catalysts still suffer from relatively low photo-catalytic efficiencies that are much lower compared to the necessities for probable realistic uses. Based on previous research investigation, probable UV–Vis-active and vis-light-active photo-catalysts included TiO2, ZnO, Fe2O3, CdS, Bi2WO6, BiVO4, Ta2O5, Ta3N5, TaON, C3N4, and so on [39]. To date, emerging high-quality SC photo-catalyst for surmount recovery of energy deficiency and environmental hazards is a great research field [36]. Despite quick progress of conventional photo-catalysts, they are still facing numerous major challenges:
-
1.
Many SCs, particularly MOs, can absorb UV light because of their wide BG [40]
-
2.
A few SCs are not appropriate for entire H2O splitting, due to their inappropriate band location and because they only show either H2O oxidation or reduction activity [41]
-
3.
In relocation of photo-generated charge carriers to surface reactive sites, charge recombination happens simply for bulk and on photo-catalysts’ surface [42]
-
4.
The majority of bulk SC reaction active sites cannot be exposed to surface and are utilized in the photo-catalytic process [29]
Therefore, key issues to attain an excellent photo-catalytic performance depend on normal mean of high-efficiency photo-catalysts. In recently discovered new potential photo-active materials, 2DMs got much consideration. Bearing in mind various characteristics and advantages, the promising 2DMs with suitable energy band configuration can stimulate new visions [14, 43, 44]. Recent research in 2DMs has advanced the modernized attention in p–n junction; the oldest electrical mechanism was employed in electronics and optoelectronics devices research. The 2DMs offer an amazing flexibility to propose a novel (p-n)-junction device configuration, not workable through usual 3D bulk SCs. The 2DMs signify a promising category of materials that have NSs-like configuration with thickness of just one or few atoms [45]. Attempts were ignited through innovation of graphene (G) in 2004, a single-layer (SL) carbon material along outstanding thermal, mechanical, and electrical characteristics [46]. Ever since, a range of G-like 2D photo-catalysts were become a relevant topic in photo-catalysis field. The 2D photo-catalysts showed special chemical and physical properties in contrast to their bulk counterparts. Emerging 2DMs with unique structural and electronic properties and appropriate band structure have showed huge potential of achieving the desired photo-catalytic efficiency. There are numerous features which influence the photo-catalytic efficiency of photo-catalysts, e.g., composition, BG, crystallinity, surface state, and morphology of SC materials, and interfacial properties of components for composite photo-catalysts.
In view of necessities for competent light adsorption and photo-generated carrier separation and transport, if possible, a photo-catalyst must contain an elevated specific surface area (SSA), good crystalline structure, stability, and an appropriate band structure [29]. The 2DMs arrangement can supply huge SSA and a large fraction of low coordinated surface atoms to produce further UV light, whereas photon absorption in bulk materials or nanoparticles (NPs) is frequently inadequate through transmittance of light and reflection at grain boundaries [47]. Furthermore, as a result atomic size thickness significantly decreases the migration distance; charge carriers produced in 2DMs interior will be quicker to transfer on surface compared to bulk materials (Fig. 1a). It will significantly decrease recombination chance of photo-generated carriers and support photo-catalytic method. Finally, regarding surface redox reactions, distinctive 2DMs configuration along elevated ratio of surface atoms to whole atoms can cause new SAS to speed up the reaction development. Additionally, atomic breakdown energy develops into comparatively minute when thickness decreased to atomic level and so additional surface defects will come into view. These surface defects will promote and improve target molecule adsorption to make strong interaction, easy charge transfer, and better activation procedure. Photo-catalysts with such characteristics and 2DMs configuration receive high interest, and a great number of related studies were performed [32].

Adapted with permission from Ref. [48]
a Periodic table shows discovered 2DMs. b Temperature and vapor pressure relations for a variety of AZ substances. c Scheme for conversion of non-vdWs solids to 2D vdWs TMDCs, where non-vdWs solids like MAX phases are gradually transferred to 2D TMDCs through a topological conversion response (MAX + HyZ (gas) → AZ + MZ), related to volatile AZ products.
To concentrate on these challenges, formations of new and more competent photo-catalysts are required to energetically investigate in this field [49]. The 2D structures along with foreign electronic properties and a high SSA are formed from layered [50] and non-layered [48] materials. The layered materials are identified through strong in-plane bonds and weak van der Waals (vdWs) force in layers. On the other hand, Ajayan and co-workers [48] recently discovered an efficient formation approach via the progressive conversion of non-vdW solids to 2D vdWs transition metal chalcogenide (TMDCs) layers with recognized 2H (trigonal prismatic)/1T (octahedral) segments (Fig. 1b, c). Conversions, obtained after exposing non-vdWs solids to chalcogen vapors, were controlled utilizing enthalpies and reaction products vapor pressures. Heteroatoms-substituted (e.g., phosphorus and yttrium) TMDCs were also formed by the same scheme, so a general formation scheme is allowed to form phase-selected TMDCs’ 2D configurations with excellent stability at elevated temperatures (about 1373 K) and obtain scalable manufacture of SLs. These 2D TMDCs have wide uses in catalysis, electronics, and energy storage applications. On account of remarkable structure-based, chemical and physical, properties of 2DMs, the construction of few-layer (FL) or single-layer (SL) 2DMs provokes broad attention as talented photo-catalysts with numerous benefits:
-
1.
The BG and light absorption of 2D-SC can adjust via altering layers number [51].
-
2.
The (e−–h+)-recombination in case of bulk can decrease because of atomic size of 2DMs [52].
-
3.
The SSA of the SCs is significantly enhanced, and most of the SASs can be exposed at surface and included in photo-catalytic reaction [47].
Along with the different 2D photo-catalysts with only FLs or SL structure, G-based photo-catalysts, 2D oxides, 2D chalcogenides, 2D graphitic carbon nitride (g-C3N4), and other 2D-SCs started gaining huge attention in photo-catalysis [49]. Although 2D photo-catalysts are viewed as talented materials to exchange solar energy into chemical energy as H2-formation, there are a number of hurdles, which limit their uses [49], as follows:
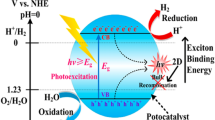
-
1.
The exciton binding energy in 2DMs-based photo-catalysts was significantly enhanced because of smaller unfavorable e−-screening than bulk material [53].
-
2.
Some 2DMs-based SCs are not stable in aqueous solution or air; thin-layer 2D SC can be assembled collectively or oxidized through photo-generated h+s during reaction, which leads to deterioration in photo-catalytic activity [54].
-
3.
While (e−–h+)-pairs recombination is less compared to bulk SCs, it still resides in 2D photo-catalysts [55].
-
4.
Reduction potential and oxidation potential of few 2D-SCs are not enough in overall H2O splitting [55].
To address such issues, a range of approaches were designed to increase photo-catalytic activity of 2DMs-based photo-catalysts, for example, doping with a metal or nonmetal elements, inducing defects, and coupling with metal or SCs, which will be discussed in detail in the upcoming sections [56]. In fact, photo-activity of photo-catalysts relies on their properties, for instance, electron affinity, crystal structure, BG, and interface in photo-catalyst as well as co-catalyst [57]. Consequently, for well-organized transfer of H2O to H2, the mixing of photo-catalyst and co-catalyst required novel interface structure. This kind of interface can optimize absorption of light for photo-catalysts and support e−/h+ separation. Normally, bigger contact area at interface can offer enough charge transfer and trapping channels for parting (e−–h+)-pairs generated by incident light [49]. The above-mentioned problem has one another solution, which is the hetero-structure formation of 2DMs. In contrast to 0D–1D, 1D–1D, 0D–2D, and 1D–2D interfaces, 2D–2D coupled hetero-structure-based interfaces concerned broad concentration in photo-catalysis due to their particular advantages, as follows [49]:
-
1.
The creation of intimate interface in two SCs is in support of exciton dissociation, which enhances the photo-catalytic quantum efficiency [58].
-
2.
It is simplistic and proficient to structure the intimate interface in 2DMs-SCs, even if they have some mismatch of lattices [59].
-
3.
Large lateral size along with high SSA leads to huge contact area in 2D/2D photo-catalysts that advance (e−–h+)-pairs’ separation and transfer [60].
-
4.
The band potential coordinated to overall H2O splitting by integrating H2/O2-evolution photo-catalyst. Therefore, oxidation and reduction influence of SCs is balanced for H2O splitting [61].
-
5.
Creation of 2D/2D hetero-structure is advantageous to develop stability of photo-catalyst because of increase in photo-corrosion and agglomeration [62].
As a sustainable technology, the SC photo-catalysis has gained significant attention in the recent decades due to possible ease/resolve energy and environmental pollution concerns. Therefore, due to 2D/2D interface advantages, many 2D/2D structures are formed recently to improve photo-catalytic performance of photo-catalysts [63, 64]. Based on these advantages, we are going to summarize most of the related topics, which can further improve the photo-catalytic phenomenon for H2O splitting (H2O oxidation and H2 evolution), CO2 reduction, N2 fixation, organic production, removal of pollutants based on G-based photo-catalysts, 2D oxides, 2D-chalcogenides, 2D g-C3N4, and some other 2D-SCs.
The 2DMs reviewed here are considered as low-dimensional materials with thickness ranging from SL to few nanometers (nm) by means of basal plane controlling total surface area, and 2DMS-based SCs photo-catalysis principles, synthesis, and stability will be briefly reviewed. Here, up-to-date development of 2DMs-based photo-catalysts is summarized, and significant evaluations of categorizing and convenient production method of 2DMs-based photo-catalysts are presented. To further boost these results, different policies to engineer electronic structure of 2DMs-based photo-catalysts are summed up, such as component tuning, thickness tuning, defect, and doping engineering. Hybridization with insertion of outside components and keeping 2D structure is explained to improve photo-catalytic efficiency, for example, quantum dots (QDs)/2DMs, single atoms/2DMs, molecular/2DMs, and 2D–2D stacking materials. Therefore, we will give a concise explanation of recently developed 2DMs, their applications in photo-catalysis, and the promising approaches for the photo-activity progress from the perspective of chemical doping, hetero-structure layout as well as functional structural design assembly. More importantly, attention will be paid to advancement of versatile photo-catalytic applications of 2DMs-based photo-catalysts in H2O oxidation, H2 evolution, CO2 reduction, N2 fixation, organic synthesis, and elimination of pollutants. Besides, manufacture approaches and characterization methods of 2D/2D photo-catalysts are also reviewed. Finally, ongoing opportunities and challenges for upcoming progress of 2DMs-based photo-catalysts in this exhilarating yet still upcoming area of research will be projected [29, 32, 49] and a short summary of present research position and challenges, with respect to 2DM-based photo-catalysts for photo-catalysis applications, will also be explained [29]. It is extremely important and insistent to present a timely updated widespread review on this matter to endorse further progress in the upcoming direction [32].
2.3 Benchmark Photo-catalysts
As it appears that too long step is in our conviction also a little that considered in future investigations that are association derived from present price of photo-catalytic materials. This feature looks alienated to laboratory-level work, deals with elementary information, and consequently accords with the rule not to be hampered by funds restrictions. On the other hand, we must admit that exploration is increasingly related to industry, and funding is governed through financial analysis of project, so this feature can no longer be ignored. In recent publications, it is expected to go forward in claims on importance of reported materials due to the absence of precious metals that were still considered as co-catalysts [65]. It is a too common assumption that does not inform the features of cheaper material; consequently, not including a precious metal (frequently applies in small quantity) is not essentially an economic choice, because material still depends on expensive starting materials, or tiresome reaction conditions (purification, temperature, solvent, etc.) that finally end up in inflating the cost of the proposed catalyst. It may take time to endeavor a little quantitative cost estimation to propose photo-catalyst, not only because the time is a necessary feature, but also getting support for optimizations in this field from other researchers [66]. Coming to the point, the following three key components are proposed to be explored to compare the built-in photonic effectiveness of a variety of photo-catalyst materials in laboratories.
-
1.
Incident photon flux (photon numbers with respect to wavelength per time).
-
2.
“Optimum rate” (achieve the highest photo-catalytic rate through changing photo-catalyst quantity in a specified reactor).
-
3.
Rate of reactant expenditure or product evolution (at optimum rate under diverse reaction conditions).
The increase in heterogeneous photo-catalysis interest and other solar fuel conversion schemes will unavoidably lead to more research in this area. Unfortunately, many research works enclose imprecision while studying photo-catalytic measurements, particularly while reporting gas evolution [67]. It is taken toward benchmark materials’ selection difficulties as there is non-reliability in efficiency measurements. In the literature, some common errors are observed in expectancy of increasing overall quality this direction. It is recommended that classification for exploring photo-catalytic rate is given as follows:
-
1.
Reactant conversion kinetic or product formation rates.
-
2.
Incident photon flux with respect to wavelength.
-
3.
Activities or partial pressures of reactants and sacrificial reagents.
-
4.
Solution type, electrolyte concentration, and pH.
-
5.
Quantity of photo-catalyst, co-catalyst, and solution.
-
6.
Flow rate of reactor and volume/dimension of reactor.
Following combined experiments carried out to assess photo-catalytic performance in likely comprehensive approach, the next step is to comprehend how recently studied photo-catalyst ranks in between present photo-catalysts. Benchmark cannot recommend from all considerations mentioned previously. A lot of investigations have been inadequately performed on this significant part of work, and only contrast materials are benchmark reference catalysts, for instance TiO2 Degussa P25. It certainly offers an early essence of photo-catalysts, but all the time comparison cannot provide a good judgment and is not enough to validate the published results. It might appear understandable, but arises a first theoretical question: Is model reported activity vs. Degussa P25 still possessed nowadays? We are bombarded with a variety of novel guidelines of editors, industries, grant agencies, and so on, that it is vital that upcoming photo-catalytic studies must concentrate on utilization of vis-light irradiation. It is a logical insight that provided strong relationship of photo-catalysis with sustainability, future realistic growth should take toward green energy, and process must hinge on utilization of sun light. A lot of energy is irradiated through solar spectrum in the range of vis spectrum (43%), but still more is in fact irradiated through IR rays (52%), yet at this moment it is complicated to utilize it for SC photo-catalysis. It is confined energy to produce requisite charge separation in the majority of SCs-based photo-catalysts. However, it is valuable to note that only some ground-breaking works on exploitation of IR radiation have come forward [68,69,70,71], that hold interesting promises for future research development. Indeed, UV region (5%) is far too little, and so there is commonsense that wide BG (≥ 3.0 eV) SCs by themselves can no longer participate in leading role and become outdated unless investigated for development of strategies built around multi-component structural arrangements, for example Z-schemes and p–n junctions. Therefore, it looks conflict that activity must be indefinitely benchmarked against a UV-active SC, i.e., Degussa P25.
In these days, state-of-the-art catalysts’ table of comparisons are emerging more frequently in research work. These are more helpful, if selected with care. Tables should not evaluate one but maximum possible activity potential factors. A comparison between QY does not reveal a lot about catalyst selectivity and stability, which are the two equal significant conditions of comparison. The experiment duration choice for calculating QY is arbitrary and thus can be simply turned to researchers’ expediency, losing objective, particularly while kinetics of product formation are not steady. Furthermore, a photo-catalyst with superior AQY or QY could be simply synthetically better, as exclude a donation to evolve product through other mechanisms working in dark. One more significant feature is that AQY experiments are generally performed with monochromatic light source, and as declared already, QY differs along excitation wavelengths. Tables of comparison completely conversed on QY preferably demand of comparison depend on polychromatic lights sources or as a minimum between photo-catalysts mainly absorbing in same narrow wavelengths range (a situation hard to attain). It is obvious to compare standard catalyst and synthetic catalysts discovered under similar catalytic conditions. Terminology is an additional feature not to be underestimated, as it can be the source of perplexity. As distinct earlier QY and AQY refer to quantity of consumed reactant (or product formation), other International Union of Pure and Applied Chemistry definitions more frequently utilized in heterogeneous photo-catalysis regarded as photonic efficiency (PE) and quantum efficiency (QE):
-
QE = photochemical events/absorbed photon flux
-
PE = photo-reaction rate/rate of incident photons [72]
Researchers need confirmation whether they are comparing the same factors. In general, we discourage a benchmark prepared completely for QY, PE, or QE, as in our estimation it is imperfect and deceptive [73]. Other activity data can propose extra basic information: Reporting product formation rates over unlimited time offers evaluation of catalyst stability and not very precise suggestion of probable diverse system, as well as a comparison on such terms is necessary for designing catalysts to be formed at commercial level. Compared rates should be studied for catalyst per both surface area and mass, for cause elucidated above.
3 Classification of 2DMs for Photo-catalysts
Advancement in material and engineering science over the past years has allowed huge development in catalysis, sustainable energy production, sensor, and electronics. Novel spectroscopy and nano-fabrication techniques offered tools to comprehend primary materials’ properties and to materialize their functionalities by adjusting their configuration and composition. It leads to enormous advancement in multi-component industrial catalysts [73] (e.g., become weak after treatment [74]), excellent chemical production, electrocatalysis (e.g., fuel cell catalysts [75]), and photo-catalysis [76]. This growth was not only governed through turnover and market demands but also through elevating community understanding, rules for environmental safety, and sustainable growth. Currently rising sustainable development and technologies create utilization of a broad range of components, of which some are rare and unequally spread on earth and therefore have economic viability and at probable risk supply. In some way ironically, sustainability and risks associated with material are frequently ignored in academic-level investigation. It is due to functionality and performances during working conditions generally prevailed over synthesis and takes apart costs; hence, evaluation of material criticality and its viability is very intricate and basically goes ahead [77]. However, a basic point of view is materials’ sustainability for final target in renewable energy synthesis. Certainly, as clean energy is almost limitless (e.g., solar, wind), materials and chemicals utilized to transfer it to real electrical energy are obtain rarely. Notably, precious metals group (i.e., platinum), rare-earth elements, gallium, aluminum cobalt, and many others [78] are indispensable components of immensely utilized commercial catalysts. If dependence can be decreased via replacement, such materials would be recycled more competently in the future to circumvent economic disturbances and increasing reserved competition [79]. These materials should have the following properties:
-
1.
Catalyst durability upgrading through material design (post-modifications, confinement)
-
2.
Lowering noble/rare metal loadings, whereas upholding high activity, through maximizing active surface area (atomic-level thickness in low-dimensional materials)
-
3.
Substituting significant components with cost-efficient and abundantly accessible ones (base metals, carbo-catalysis)
-
4.
Enhancement of durability in catalysts’ synthesis and removal (green chemistry-based catalyst recycle)
-
5.
Evaluation of toxicity and environmental effect of catalyst materials
The catalysts’ nano-structure is another supreme feature to be considered as it can be used to analytically study and compare diverse catalysts to realize tendency in activity. Generally, the size of photo-catalyst materials also affects their electronic arrangement originated through quantum confinement effects (less than 10 nm) and degree of interface with support, as smaller sized photo-catalyst materials have a larger portion of atoms at metal support edge [57]. For example, Taejong Paik et al. [80] defined the optical BG increased in tungsten oxide (WOx) NWs compared to stoichiometric WO3 bulk counterpart, because of Burstein–Moss shift. This increment confirmed direct photo-catalytic H2 evolution from WOx NWs via alcohol photo-reform. The stable H2 production on platinized WOx NWs is pragmatic under conditions where platinized bulk WO3 and bulk WO2.9 powders either do not show activity or show very low rates, proposing that enhanced surface area is the answer for enhanced activity. As a result, controlled size and composition can cause unanticipated and important alterations in SC photo-catalytic materials properties [81]. As an ideal candidate for photo-catalysis, the mainly studied 2DMs-based photo-catalysts can be divided into different types: counting, MOs, metal composite oxides, MHOs, bismuth-based materials, metal chalcogenides, and metal-free photo-catalysts. Based on photo-catalyst compositions, the 2DMs used in photo-catalysts can be mainly categorized as illustrated in Fig. 2. The 2DMs can be synthesized either through exfoliation of parent layer material through top-down method or formed from small molecules using bottom-up self-assembly technique. Synthesis of 2DMs with tunable layer number, edge morphology, and degree of crystallinity is vital for utilizing these materials for elevated activity catalytic applications and is also discussed in our recently published reviews [3, 4, 19]. Therefore, in this part, we will only provide a concise introduction for basic properties with small explanations about the synthesis strategies of such three types of 2DMs, which are utilized for photo-catalysis applications.
3.1 2D metal Oxides (MOs) NSs
The MOs are broadly studied for photo-catalysts. Certainly, fabricating 2DMs-based MOs is considered as an efficient way to maximize SSA and charge migration and hence gets a competent photo-catalytic performance [32]. So far, numerous MOs with 2DMs structures have been formed and used in photo-catalysis applications, for example TiO2, Fe2O3, Cu2O, ZnO, WO3, SnO, In2O3, CeO2, HNb3O8, etc. [82]. Due to the basic non-layered structure feature, some 2D-MOs were complex to be formed by facile ultrasonic exfoliation technique from their bulk counterparts. So, numerous other means were applied for controlled formation of 2D-MOs. For example, a lamellar inorganic–organic hybrid intermediate policy was planned to form ultra-thin TiO2 NSs [82]. Utilizing Ti-isopropoxide as a Ti source, octylamine as a capping reagent, 2-phenyl ethanol as solvent, lamellar TiO2-octylamine hybrid precursors were obtained via solvothermal process (Fig. 3) [32]. The ultrasound-based exfoliation-resulted powder was washed to eliminate octylamine and get clean, ultra-thin TiO2 NSs. The AFM result showed that TiO2 NSs’ average thickness was about 1.66 nm. A lot of other types of MOs-NSs, such as Cu2O [83] and In2O3 [84], were also formed via a similar technique. Taking advantage of ultra-thin size, the enhanced density of states (DOSs) by Fermi level (FL) and enhanced charge density on TiO2 NSs surface were obtained. For this reason, TiO2 NSs displayed quick transport of carriers and therefore achieved 450 times improved photo-catalytic activity as compared to bulk TiO2 for CO2-reduction for formate fabrication. Additionally, exfoliated single-crystalline WO3 NSs were formed by Bi2W2O9. On account of layered Bi2W2O9 structure that is composed of [W2O7]2− and [Bi2O2]2+ layers, the WO3 layers were attained through careful etching of [Bi2O2]2+ layers via processing of acids like HCL and the stabilized WO3 layers can be obtained through the tetrabutylammonium hydroxide surfactant. These exfoliated WO3 NSs showed an improved BG as compared with bulk-WO3, caused by quantum confinement effect. With exception of an exfoliation method, the direct preparation development of MOs-NSs was obtained by wet chemical technique. Utilizing surfactant’ self-assembly approach through polyethylene oxide–polypropylene oxide–polyethylene oxide and ethylene glycol as co-surfactant, various MOs with ultra-thin thickness were formed, for instance TiO2, Fe3O4, Co3O4, ZnO, MnO2, and WO3 [32].
In the last four decades, various MOs, e.g., TiO2, ZnO, SnO2, WO3, and Fe2O3, were broadly examined as photo-catalysts [42, 85]. Among them, TiO2 was the most explored one due to its good stability, biocompatibility, and favorable electronic structure as well as light absorption nature [34]. The 2D-TiO2 NSs obtained from the exfoliation of layered titanate have drawn attention in utilizing them as photo-catalysts [86]. The 2D-TiO2 NSs showed SC nature similar to their bulk cousins and include rutile and anatase form of TiO2, but with somehow superior BG because of the size quantization. For instance, Ti0.91O20.36-NSs exhibited a BG of ~ 3.8 eV that was higher than that for anatase TiO2 (3.2 eV) [87]. Top-down multi-step access found on intercalation and exfoliation of layered MOs was well recognized to form MO-NSs [88]. For example, for TiO2-NSs, layered titanates were initially formed by high temperature, conventional solid-state reaction of TiO2, and mixture of alkali metal carbonates (Fig. 4).

Adapted with permission from Ref. [29]
Scheme showing the crystal structure of lepidocrocite kind titanate and its exfoliation into TiO2 NSs.
After that, it was developed with an acid solution to generate protonated intermediate by ion-exchange route. The interlayers of protonated titanate were more extended by changing protons with a definite quantity of bulky organic ions, such as tetrabutylammonium cations (TBA+). In suitable condition, layered configuration was exfoliated induced via weak shear force, for instance, mechanical shaking in aqueous solution. Different kinds of layered MOs-NSs, such as WO3, titanoniobate [89] (TiNbO5, Ti2NbO7, and Ti5NbO14), perovskite oxides [90] (K2Ln2Ti3O10, together with KLnNb2O7 and RbLnTa2O7 (here Ln stands for lanthanide ions)), HNb3O8, HCa2−xSrxNb3O10, and HCa2Nb3−yTayO10 [91], were formed through analogous solid-state reactions method and wet-chemical exfoliation methods. For instance, titanoniobate NSs have exhibited enhanced photo-catalytic performance in organic pollutant removal [92]. Recently, Tae et al. studied the formation of numerous diamond-shaped titanate NSs with a normal lateral size < 30 nm, by applying a straightforward wet-chemical technique [93]. In very recent times, Zhou et al. formed freestanding, SL Bi2WO6 MSs by wet-chemical technique via using cetyltrimethylammonium bromide. Bi-atoms on SL were not saturated; hence, introduced numerous active sites on surfaces, which generated h+s directly under light irradiation. An excellent photo-catalytic performance of SL Bi2WO6 for photo-degradation of RhB was recognized by fast charge carrier separation at highly photo-active surface [94].
3.2 Metal Composite Oxides
Compared to MOs, metal composite oxides also showed advantages to photo-catalysis, and numerous metal composite oxides were formed with ultra-thin thickness [91]. Consistent with acid/base effect and ion intercalation supported exfoliation method, HNbWO6 NSs were obtained by dispersing the layer HNbWO6·1·5H2O into tri-ethanolamine aqueous solution [95]. The results based on AFM calculations showed that HNbWO6 NSs thickness was about 1.8 and 2.0 nm, which are in agreement with SL significance. As-synthesized HNbWO6 NSs suspensions displayed a proficient activity for photo-catalytic H2-evolution with a moderate rate of 158.9 µmol h−1. Furthermore, ion-exchange approaches through utilizing ultra-thin precursor were used for synthesis of metal composite oxides. For instance, SnNb2O6 NSs were obtained through K4Nb6O17 NSs and SnCl2 as precursors [96]. Through K4Nb6O17 ultra-thin thickness, it was preserved in SnNb2O6 with ~ 3 nm thickness, as confirmed through AFM study. In comparison with bulk SnNb2O6, the SnNb2O6 NSs were having improved BG and more negative CB potential, denoting good reduction capability for photo-catalytic-based H2-evolution. Furthermore, charge transfer effectiveness in SnNb2O6 NSs was also enhanced because of ultra-thin thickness. Additional research showed that the outstanding vis-light H2-evolution activity was acquired over SnNb2O6 NSs, approximately 14 times superior to bulk SnNb2O6.
3.3 Metal Hydroxides (MHOs)
Ultra-thin MHOs were increasingly considered as significant class in 2DMs, which showed an exciting view in numerous sectors, for example catalysis, energy storage, and conversion. On account of simplicity of guideline for cations, the preferred BG was formed in MHOs by incorporating particular photo-active metal cations. So, the ultra-thin 2D-MHOs structure showed a great potential toward photo-catalytic uses. For example, ZnAl-layered double hydroxide (LDH) 2D-NSs were formed via a reverse micelle technique and used as photo-catalyst for converting CO2 to CO [97]. By means of sodium dodecyl sulfate as surfactant, 1-butanol as co-surfactant, translucent and stable reverse emulsion structure was created in an iso-octane/H2O mixed solution. Following Al and Zn sources addition to mix solution, urea was used to generate alkaline condition and formed ZnAl-LDH with ultra-thin configuration. Thickness was about 2.7 nm in standing NSs in TEM image and is equivalent to the thickness of 2D-LDHs’ layers. Due to the ultra-thin thickness, O2 vacancies (Vo) were formed in ultra-thin ZnAl-LDH NSs, resulting in the creation of Zn+–Vo complexes. The DFT-based study showed a novel defect-level hybridization with both occupied Zn 4s orbitals and O2p orbitals emerging in BG of ultra-thin ZnAl-LDH NSs compared with bulk ZnAl-LDH. The Zn+–Vo complexes can provide e− trap sites for CO2 photo-reduction. Consequently, an appreciably amplified photo-catalytic activity for CO2-reduction was obtained for ultra-thin ZnAl-LDH NSs compared with bulk ZnAl-LDH. Except ZnAl-LDH, several new MHOs with ultra-thin thickness, for example CoOOH [98], NiTi-LDH, and ZnTi LDH [99], also showed wonderful performance for diverse photo-catalytic uses.
3.4 Metal Chalcogenides
The TMDs have gained much interest because their mechanical, optical, and electrical characteristics were explored for a wide range of applications, for instance biosensors, catalysis, lithium battery cathodes, transistors, memory devices, photovoltaics, photodetectors, photo-catalytic solid lubricants, and PEC conversions. The TMDs (e.g., MoS2, WS2, and TiSe2) are a large group of layered materials with common representation as “MX2,” where M is a transition metal element of group 4–10 ((Ti, Hf, Zr), (Ta, V, Nb), and (W, Mo)) and X is the chalcogen atom (S, Se, Te). The TMD NSs have different functions in PC and PEC applications. They behave as a photo-sensitizer via increasing light harvest ability in vis region of sun irradiation, a charge separator throughout appropriate energy band arrangement, and a charge carrier. Correct function of 2D nanosheets (NSs) depends on utilization of reaction scheme. In consequence of special electronic configuration, in general metal chalcogenides showed a comparatively broad light absorption area (Fig. 5), which were measured to be a group of talented materials having photo-catalytic uses. Normally, the stoichiometry of TMDs can be expressed through formula MX2, in which M and X signify a chalcogen and a transition metal, respectively. The single layer of a TMD involves three atoms, where M is situated in two X (Fig. 5a, b). The configurations of 1T and 2H phases of MoS2 are shown in Fig. 5c.

Adapted with permission from Ref. [100]
a Transition metals and three chalcogen elements (yellow color) which are composed of TMD layer structures. b TMD structures. c MoS2 two-phase (1T, 2H) structures.
Recently, many 2D metal chalcogenides have been formed and showed an excellent photo-catalytic performance, e.g., CdS, MoS2, SnS2, SnS, In2S3, ZnIn2S4, ZnSe, and SnSe [101]. The synthetic techniques of such 2D metal chalcogenides generally concentrate on exfoliation, because of essential layer structures. Taking hexagonal SnS2 (h-SnS2), such as h-SnS2 SLs, can be attained through refluxing bulk-SnS2 in formamide to rupture interlayer vdWs’ interactions [102]. Almost transparent aspect of SnS2 NSs in TEM image exposes ultra-thin thickness, and it was described to be 0.61 nm via AFM, well matched with SL-SnS2 slab along [001] direction. As a result of SL configuration, electronic structure of SLs-SnS2 experienced discrete changes, with increased BG, higher DOSs at VB edge, and faster interfacial charge transfer. Therefore, SLs-SnS2 delivers a surprisingly improved photo-catalytic H2O splitting activity (70 times development) compared to bulk-SnS2, under vis-light. The 2D metal chalcogenide NSs, for example MoS2 [103], SnS2 [104], TiS2 [105], WS2 [106], MoSe2 [107], WSe2 [103], etc., are rising as a new significant class of 2DMs in the photo-catalysis applications because of their good electronic properties [108]. Taking MoS2 as an example, bulk-MoS2 materials have indirect BG of 1.2 eV that is not suitable for photo-catalytic reactions caused by the lack of oxidation or reduction potential (E0) for activating photo-catalytic method. However, MoS2-NSs had been established with having a direct BG of ~ 1.96 eV because of quantum confinement effect that provides MoS2/-NSs along appropriate band positions and capability for vis-light absorption. In contrast to most of the layer MOs, vdWs bonding of metal chalcogenide interlayers creates exfoliation of these layers easily. Until now, a lot of top-down approaches are described for the formation of SL or FL metal chalcogenide NSs, for example lithium intercalation–exfoliation, mechanical exfoliation, and liquid phase ultrasonic exfoliation [109]. Furthermore, bottom-up chemical production and chemical vapor deposition (CVD) techniques suggested potential influential alternatives such as exfoliation techniques for fabricating metal chalcogenide NSs. For example, Cheon et al. introduced disk-shaped ZrS2 NSs with < 2 nm thicknesses and lateral size ranging from 20 to 60 nm via reacting ZrCl4 and CS2 in oleylamine [110]. This method was, soon after that, extended for other transition metal selenide and sulfide NSs. The MoS2-NSs were formed via solvothermal techniques using (NH4)6Mo7O24·4H2O and thiourea as precursors [111].
3.5 Bismuth-Based Materials
Recently, bismuth (Bi)-based materials have been broadly investigated and studied for their photo-catalysis applications because Bi6s in Bi(III) can hybridize with O2p orbitals to generate novel favorable hybridized VB and BG of Bi-based materials which are narrowed for absorption of vis-light. Due to continuous improvement in photo-catalytic performance, numerous Bi-based materials with controlled ultra-thin thickness are formed, for example Bi2WO6 [112, 113], Bi2MoO6 [85], BiVO4 [114], Bi2SiO5 [115] (BiO)2CO3 [94], Bi3NbO7 [116], BiOX (X = Cl, Br, I) [117], and Bi2O3/Bi2O4−x nano-composite [118]. The SL Bi2WO6 NSs were formed via surfactant cetyltrimethylammonium bromide (CTAB)-supported hydrothermal technique [94], where Br-ions from CTAB were adsorbed at SL Bi2WO6 surface and produced Coulomb repulsion forces, which delayed stacking of SLs Bi2WO6. Furthermore, hydrophobic long-chain cationic CTA+ at Bi2WO6 surface supplied an extra surface repulsion to further stop crystal growth along the c-axis. Therefore, SL Bi2WO6 slab (0.8 nm thickness) with [BiO]+–[WO4]2−–[BiO]+ sandwich substructure was achieved, as supported from AFM analysis (Fig. 6a–d). Plentiful coordinative unsaturated Bi-atoms were exposed at SL Bi2WO6 SNs and act as active sites. After irradiation with light, h+s is produced in [BiO]+ as e−s in [WO4]2−. Resembling hetero-junction interface, sandwich [BiO]+–[WO4]2−–[BiO]+ substructure permits efficient interface for space charge separation. Therefore, SL Bi2WO6 displayed significantly improved photo-catalytic activity toward pollution deduction in vis-light.
a Bi2WO6 crystal structure. b Fabrication method of the SL Bi2WO6 with CTAB support. c TEM/HR-TEM of Bi2WO6 formed by CTAB support. d AFM analysis of SL Bi2WO6 based on CTAB. Adapted with permission from Ref. [32]. Comparison of photo-catalytic degradation of e MB, f MO, and g phenol over Bi2O3, Bi2O3/Bi2O4−x, and Bi2O4−x. Adapted with permission from Ref. [118]
In addition, as-synthesized SL Bi2WO6 illustrated H2-evolution activity under vis-light, even if Bi2WO6 nano-crystal in fact holds no H2-evolution activity. Altering the surfactant type to polyvinylpyrrolidone (PVP), ultra-thin BiOCl NSs were attained by means of a solvothermal treatment [119]. The BiOCl nanoplate’s thickness (≈ 30 nm) was reduced to 2.7 nm, while PVP was applied as a capping agent, as verified through the AFM analysis. The polyvinyl skeleton structure of PVP prevented more development of the BiOCl nano-crystal by generating passivation layer about BiOCl cores via strong interaction with Bi3+, N, and O atoms of pyrrolidone ring. This suppressed the agglomeration of BiOCl nano-crystal along c-axis during repulsion forces in between polyvinyl groups. Therefore, ultra-thin BiOCl NSs were prepared via the PVP-assisted solvothermal treatment and this method was further used for synthesis of ultra-thin BiOBr and BiOI 2D form [120]. The attained ultra-thin thicknesses give BiOCl NSs with upshifted CB and VB potentials and reduced the BG relative to BiOCl nano-plates. As such, effective division of photo-induced (e−–h+)-pairs was obtained and caused an increase in photo-catalytic activity for pollutant removal [32]. Paolo Fornasiero and co-workers also explored Bi2O3/Bi2O4−x composite that functions as a potential photo-catalyst (Fig. 6e–g). The aim of the study was to begin active species on photo-catalyst surface via utilizing a non-traditional advancement. Therefore, they utilized (UV–Vis)-light to stimulate alterations in Bi2O3 surface that produces Bi2O3/Bi2O4−x nano-composite arrangement. So, for methylene blue (MB) such surface modifications bring significant enhancement in photo-catalytic performance. The wide BG with respect to Bi2O3 along excitation considerations proposes that analogous photo-induced crystal modifications, although exist, should be insignificant for TiO2-based materials. Until now, only careful designed thermal treatments were able to make exciting anatase/rutile nano-composites with outstanding photo-catalytic performance [118].
3.6 Metal-Free NSs
Excluding metal containing SC 2DMs, metal-free 2DMs were also formed as photo-catalysts. Recently, new classes of metal-free 2DMs have been introduced from lightweight and abundant elements, such as carbon, phosphorus, and binary carbon nitride, boron carbide, and hexagonal boron nitride (h-BN) that reveal new prospects for photochemistry. The 2D-G with hexagonal sp2-hybridized structure is inspiring great research concern in a range of energy-related uses because of its elevated carrier mobility. The high flexibility and larger SSA alone from accessibility of solution processable graphene oxide (GO) allows 2D-G NSs to simply merge with other SCs to form electronic bridges [121]. Recently, 2D-G have been intensively studied in photo-catalytic fields and demonstrated as competent e−-acceptor to improve the charge transfer and reduce (e−–h+)-pair’s recombination to improve photo-catalytic activity of composite photo-catalysts [3, 4, 18, 19]. More interestingly, although SL 2D-G is recognized as a semimetal with a zero BG, which is not suitable in light absorption, numerous scientists have confirmed that functionalized 2D-G base analogy like 2D-GO could be promising materials for nonmetal photo-catalysts as band structure of GO is associated with its degree of oxidation that can be engineered via choosing appropriate preparation methods. For example, Yeh et al. observed a 2D-GO that could work as active photo-catalyst in H2O splitting [122] and can gradually produce H2 from 20 vol% methanol solution in H2O and pristine H2O after irradiation with UV/Vis-light. After that, Hsu et al. studied 2D-GO and showed an elevated photo-catalytic efficiency for transformation of CO2 to methanol (CH3OH) by solar light irradiation [123]. As an equivalent of 2D-G, g-C3N4 NSs were rapidly rising because of their excellent chemical and electronic properties [124]. Bulk g-C3N4 has a layered 2D configuration and proper BG (~ 2.7 eV) for light absorption in visible range. The g-C3N4 NSs were obtained through delaminating bulk layered g-C3N4 that is usually formed via pyrolysis of N2-rich precursors through bulk reaction or polycondensation.
A new metal-free photo-catalyst, with outstanding photo-catalytic proficiency of g-C3N4 NSs under vis-light irradiation, was verified in many photo-catalytic uses. For example, Niu et al. [125] studied a simple top-down approach to form g-C3N4 NSs via oxidation etching of bulk g-C3N4 in air under high temperature (Fig. 7). The acquired g-C3N4 NSs thickness was about 2 nm with SSA 306 m2 g−1, which was high in comparison with bulk phase. Quantum confinement effect causes enhanced e−-transfer ability toward in-plane direction, and the lifetime of photo-generated charge carriers was improved. Therefore, photo-catalytic performance of g-C3N4 NSs for H2-production process was really enhanced. In recent times, other liquid phase exfoliation techniques are formed to synthesize g-C3N4 NSs from bulk counterpart. For instance, Yang et al. prepared freestanding g-C3N4 NSs through liquid phase exfoliation of g-C3N4 powder in isopropanol; this exhibited good photo-catalytic effectiveness for H2-evolution by applying vis-light irradiation. Photo-catalytic efficiency of exfoliated NSs was higher > 17 factor contrast to non-exfoliated counterpart and with a factor of > 8 than already described g-C3N4 NSs [126]. Apart from distinctive energy band configuration, g-C3N4 is more active toward many photo-catalytic uses, for example H2-evolution, CO2-reduction, pollutant deduction, disinfection, etc. As a result of in-plane graphite-like layer configuration with strong C–N covalent bonding and interlayer weak vdWs’ forces, bulk g-C3N4 was accountable to be exfoliated and obtained in FL or even SL form. Generally, there are two methods for g-C3N4 exfoliation, i.e., thermal oxidation and ultra-sonication-based liquid exfoliation techniques. In view of that, H2-bond coherent strands of polymeric melon units in layers were not sufficiently stable beside oxidation. Liu et al. [125] formed a thermal oxidation exfoliation method to form an ultra-thin g-C3N4 NSs. Thicknesses of bulk g-C3N4 were steadily reduced with increasing times through layer-by-layer etching method (Fig. 7b–d). After 120 min, thermal oxidated g-C3N4 NSs with almost 2 nm thicknesses were obtained. Since quantum confinement effect and increased BG promoted e−s migration rates along in-plane direction, H2-evolution activity improved 5.4 times, as compared to bulk counterpart. Encouraged by this synthesis method, numerous advance researches achieved organizing ultra-thin g-C3N4-NSs via modified techniques [127, 128]. The ultra-sonication assisted liquid exfoliation was observed as another effective technique to attain ultra-thin g-C3N4 NSs because of fundamentally layered structure.
In exfoliation process, the efficiency is affected due to surface energies, and when solvent and bulk materials match each other, exfoliation was extremely good. The calculated g-C3N4 surface energy was almost 115 mJ m−2, in a good agreement with H2O (~ 102 mJ m−2). Thus, with the use of H2O in g-C3N4 liquid exfoliation, exfoliated NSs with almost 2.5 nm thickness were acquired [129]. Based on the analogous law, Ajayan and co-workers [130] calculated g-C3N4 exfoliated by isopropanol. The as-formed g-C3N4-NSs were having homogeneous thickness of ~ 2 nm. This ultra-thin thickness of g-C3N4-NSs displayed an improved BG as well as charge migration rate in contrast to bulk g-C3N4. This caused a 9.3 times higher photo-catalytic-based H2-evolution. Similarly, other solvents were also searched to form ultra-thin g-C3N4 and obtained improved photo-catalytic performance [131]. Despite g-C3N4, other metal-free materials were also formed, which act as photo-catalysts for different uses [132]. For example, ultra-thin silicon NSs controlled synthesis via molten salt-induced exfoliation and chemical reduction of natural clay [133]. Ultra-thin silicon NSs (≈ 5 nm thickness) showed an excellent H2-evolution performance from a H2O methanol mixture. Advanced investigations showed that proficient H2O splitting was obtained over ultra-thin silicon NSs lacking addition of co-catalyst or sacrificial agent [134]. Unfortunately, ultra-thin silicon NSs have experienced serious activity decline by extended time. How to approve suitable approach to increase stability might be heart of H2O splitting over ultra-thin silicon NSs [32].
3.7 Other Metal Containing 2D-NSs
In addition to aforementioned different types of materials, other recently introduced 2DMs, for example layered metal oxy-nitride and oxy-halides, and metal carbides, also have a great potential for photo-catalysis uses after chemical doping or combining with other SCs. For instance, bismuth oxy-halides (BiOX, X = Cl, Br, and I) were gotten increasing interest because of their outstanding photo-catalytic nature, that are analogous to or even greater than those of the anatase TiO2 [119]. Moreover, neutral layers of the Ti3C2(OH)2 formed through HF-assisted exfoliation of metal carbides, for example Ti3AlC2, were verified as competent photo-catalyst for adsorption and photo-catalytic decomposition of organic molecules in an aqueous atmosphere [135].
3.8 2D/2D Hetero-structures
Properly developing the 2D/2D hetero-structures confirmed the most talented form for further boosting the photo-catalytic activity, because of that hetero-junction interfacial effect [136]. The hetero-junction interfacial effect can encourage separation and therefore extend lifetime of the photo-generated (e−–h+)-pairs in catalyst that directly or indirectly contributes to redox reaction of photo-catalytic H2-production or organics degradation. Several attempts were applied to engineer 2D-component or reinforce the interfacial acting force to form the capable 2D/2D photo-catalysts. Although hetero-junction found on a range of dimensions (e.g., 2D/2D, 3D/3D, and 2D/3D) with exposed interface put right contact, they are all possible efficient catalysts. The 2D/2D hetero-junctions have different advantages for catalysis, as follows:
-
1.
High catalytic active sites because of great SSA/interface area and ultra-thin thickness.
-
2.
Charges are easily transferred because of small basic resistance and short transport path in 1D of the ultra-thin 2D components.
-
3.
Transparency consequence from ultra-thin thickness is helpful in light absorption.
Therefore, plan as well as the use of 2D/2D layered hetero-structures has rapidly become the most up-to-date research topics. Recently, the family of ultra-thin, 2D layered materials formed significantly. Further, other than presently used ultra-thin 2D-NSs, for example 2D-G, TMDCs, and noble metals, there are many other 2D-NSs with changeable electronic and physical properties formed from the last few years, e.g., MOs, h-BN, black phosphorus, metal–organic frameworks (MOFs), organic crystals, and covalent organic frameworks (COFs) [136].
4 Electronic Structure Engineering
Engineering an electronic configuration, the properties of ultra-thin 2DMs are experiencing a different variation, offering probabilities to enhance or even provoke novel photo-catalytic activity. There are a range of ways to engineer the electronic structure of ultra-thin 2DMs, e.g., thickness tuning, component tuning, defect engineering, doping, and so on. Now, we will explain most of them in detail in the next section.
4.1 Component Tuning
For photo-catalytic employment, component of SC establishes band configuration. As reactivity of the photo-generated e−s and h+s toward resultant surface redox reactions was usually defined by band edge potentials, component of SC demonstrates a vast effect on photo-catalytic performances. Particularly, for ultra-thin 2DMs, the electronic configuration strongly relies on equivalent constituent. Maeda et al. [91] formed HCa2−xSrxNb3O10 and HCa2Nb3−yTayO10 NSs with restricted energy band structure via interlayer exchange K+-ions with protons in layered KCa2−xSrxNb3O10 as well as KCa2Nb3−yTayO10 and after that more undergo exfoliation. By gradually engineering atomic component in NSs, the optical absorption of materials is deeply suffered (Fig. 8).

Adapted with permission from Ref. [137]
a–c TEM results of TBA+-exfoliated Ca2−xSrxNb3O10-NSs. d, e Raman spectra and UV–Vis diffuse reflectance spectrum of restacked HCa2−xSrxNb3O10 and HCa2Nb3−yTayO10 NSs.
Thoroughly, commencement of absorption edge in HCa2−xSrxNb3O10 showed a clear redshift with improved Sr-content, accompanying via BG energy reduced from 3.59 (x = 0) to 3.40 eV (x = 2). Additionally, onset of blueshift absorption edge was determined for KCa2Nb3−yTayO10 with higher Ta contents. The substitution of Nb5+ by Ta5+-ions caused more negative CB potential, which was liable for blueshift of the onset absorption edge. Thus, tunable light absorption performance was obtained by altering component and further showed an important effect on the photo-catalytic H2-evolution reaction. By utilizing an analogous plan, energy band structure of ternary sulfides, H2xZn1−xIn2S4, was efficiently engineered with ZnIn2S4 and showed optimal photo-catalytic H2-evolution rate [138]. Another significant case was tuning of halogen/O2 ratio in the bismuth oxy-halide to tune electronic structures. In accordance with DFT calculations, VB top of bi-oxy-halide was mainly composed of O2p and Xnp (n = 3, 4, and 5 for X = Cl, Br, and I) hybrid orbitals, while CB was primarily comprised of Bi6p orbitals. In the course of engineering O, X elements, BG and band edge potentials were efficiently engineered. Thus far, a number of Bi oxy-halide NSs with tuned O, X component were synthesized, for example Bi12O17Cl2 [139], Bi4O5Br2 [140], Bi4O5I2 [141], and so on. In general, with decreasing Br or Cl content, acquired materials showed narrowed BG than that of resultant BiOCl or BiOBr and reduction of I content resulted in an increase in BG comparative to BiOI. Benefiting from energy band structure range, tuned bi-oxy-halide ultra-thin NSs are showing superiority toward various photo-catalytic employments.
4.2 Thickness Tuning
SC thickness is an important factor to an electronic structure engineering and photo-catalytic performance optimization. As a result of known quantum confinement effect, BG of SC undergoes an increase, when materials thickness is reduced. Moreover, surface effect is aggravated as thickness reduced to an atomic size. The electronic DOSs can improve at the surface of ultra-thin 2DMs in comparison with interior of bulk materials. Such characteristics showed a significant impact on photo-catalytic efficiency of ultra-thin 2DMs. Based on thermal oxidation etching way, ultra-thin C3N4 NSs with thickness of ~ 2 nm were formed [125]. As thickness reduced to atomic size, electronic structure of C3N4 suffers from major difference. Consequently, due to quantum confinement effect, BG increased from 2.77 eV for bulk C3N4 to 2.97 eV in ultra-thin 2D-NSs. Simultaneously, thickness reduction grants guarantee to improve an electron transport capability toward in-plane direction and enhanced duration of photo-generated charge carriers.
Additional research originates in which CB edge of ultra-thin C3N4 NSs showed upshift in comparison with bulk counterpart [142]. So, photo-generated e−s holds strong reduction capability and helps in improving the photo-catalytic H2-evolution performance. Despite C3N4, electronic structure tuning is gained within other SCs through thickness engineering. Through thickness reduction of bulk SnNb2O6 to 50 and 3 nm, resultant BG increased from 2.30 to 2.35 and 2.43 eV, respectively, causing upshifting of CB edge [96]. It is suggested that thickness engineering was an efficient way to alter energy band configuration of SCs. Moreover, when bulk materials thickness is regularly decreased to an atomic size thickness or even SL, the ratio of exposed surface atoms to whole atoms can be prominently improved. Lack of nearby atoms formed plentiful coordination on unsaturated surface atom with dangling bonds and is leaning to bond with other atoms to attain stability. So, these surface atoms displayed a high surface energy and chemical reactivity. The free-standing SnSe and SnS NSs with all exposure surface atoms were acquired through exfoliating their bulk counterparts in mixed solvent of H2O and ethanol [143]. Taking SnS as an example, with large-area NSs resembling morphology with lateral size of almost 500 nm, their transparent properties were studied by TEM investigation, showing ultra-thin thickness of as-synthesized material (Fig. 9). The average height of SnSe NSs, calculated by AFM, was about 0.57 nm that agrees with thickness of half unit cell. Hence, these SL-exfoliated NSs calculated band structures which proposed a change from an indirect SC in bulk SnS to direct SC for SnS SLs. In comparison with bulk SnSe, enhanced DOSs were obtained at VB edge of SL SnS, which were enabling SL SnS with improved carrier transport efficiency. To get benefit from SL structure, SLs SnS showed improved photo-absorption and charge separation efficiency and later supported H2O splitting performance.

Adapted with permission from Ref. [144]
a, b TEM and HR-TEM, c selected-area electron diffraction. d–f AFM image, height distribution, and height profiles. f (1–3) stands for 1–3 in d. g Colloidal H2O/ethanol (1:1) dispersion of the as-synthesized products showing Tyndall effect.
4.3 Doping
The appropriate doping was observed as a competent approach for engineering physicochemical property of 2DMs. Conscious manufacturing of extrinsic metal or nonmetal species into SC lattice presents the prospect to adjust electronic or surface configurations of host material for enhancing photo-catalytic performance. Types and allocation of dopants are very important to control properties of host SCs. Advantages of atomic size of 2DMs; doping perhaps is a very sufficient plan to influence properties of ultra-thin 2DMs. Normally, doping always happens on bulk materials’ shallow surface due to lack of atoms accessing gallery that demonstrated a small manipulation on their total performance. Concerning ultra-thin 2DMs, atomic thickness permits efficient doping of dopants and just needs small diffusion penetration. So, it is enviable to tailor heteroatom into 2DMs and builds high competence methods.
4.3.1 Metal Doping
Metal ions incorporation into crystal lattice causes a rise in impurity levels in SCs forbidden band. Onset light absorption edge redshift is frequently examined that is recognized to cause a transition of impurity quantities to CB or VB. Normally, the very capable doped photo-catalysts primarily depend on doping alteration of metal ions that satisfied the criteria, i.e.,
-
1.
The e−s and h+s can be trapped through dopant and make sure efficient confined separation.
-
2.
The captured e−s and h+s are generated and transferred on surface successfully.
Xie and co-authors [145] doped In2S3 NSs with Co to optimize photo-catalytic H2O splitting. Through a lamellar inorganic–organic hybrid intermediate approach, Co-doped In2S3 NSs (0.59 nm thick) along with 3-atomic layers thickness were formed (Fig. 10). The electronic configuration of In2S3 using three atomic layers and Co-doped In2S3 was first verified via DFT simulations. To simulate existence of Co-dopant, certain ultra-thin In2S3 NSs with noticeably enhanced DOS are developed at conduction band maximum (CBM) as compared with perfect, ultra-thin In2S3 NSs. Additionally, Co-ion doping provides a Co-doped In2S3 material with numerous other energy levels that were resultant from Co3d splitting. Under light irradiation, e−s was simply excited through d → d internal transition of the Co-ions in tetrahedral coordination, allowing generation of more photo-generated (e−–h+)-pairs. These results were confirmed through light absorption difference of In2S3 NSs and Co-doped In2S3 in (UV–Vis)-diffusion reflectance spectrum. Considerable advancement in light absorption was viewed from 600 to 2000 nm that was consigned to creation of dopant energy levels of Co. To get advantages from doping of Co, photo-generated charge separation effectiveness enhanced about 25-fold increase in average recovery duration, as practiced through an ultrafast transient absorption spectroscopy (UTAS). Therefore, Co-doping permits 10 times developed photo-catalytic activity for H2O splitting compared to perfect In2S3 NSs. Similarly, other metal elements were also utilized for doping to engineer electronic structure of ultra-thin 2D-hosted photo-catalyst, for instance Pt, Rh, Cr, Fe, Cu, and so on [146]. For example, Fe was doped into ultra-thin BiOCl NSs, which extended light absorption range from UV to Vis-light. Photo-catalytic activity for pollutant removal and H2-evolution was increased. These results certainly verified that metal element doping is an efficient way to tune electronic structure of ultra-thin 2D photo-catalysts and can promote photo-catalytic performance.

Adapted with permission from Ref. [147]
a Representation of the formation of Co-doped In2S3 three atomic layers. b–g Characterizations for Co-doped In2S3 three atomic layers: b HAADF-STEM image and EDS mapping of an individual Co-doped In2S3 three atomic layer, c–e TEM images and HR-TEM image, f, g AFM image, and height profiles. h DOSs of Co-doped In2S3 three atomic layer slabs and i ideal In2S3 three atomic level slab.
4.3.2 Nonmetal Doping
Concerning nonmetal elements doping, two direct theories were suggested to modify an electronic arrangement and hence influence photo-catalytic performance. First, dopants can generate localized states between forbidden bands and one advantage is fusing of dopant-occupied positions with VB and upshifting of valence band maximum (VBM). Such two diverse techniques normally originate from different doping types, where surface doping will cause development of localized states and uniform doping will promote VBM [148]. As mobility of h+s in localized states is slow and after that restricts photo-catalytic efficiency, offering uniform allocation of dopant to upshift VBM and encourage h+s relocation is preferred much. The atomic thickness of the 2DMs facilitated a uniform doping because little doping depth is specifically needed [149]. By tailoring O2-atoms for 2D ZnIn2S4 NSs to replace sulfur atoms lattice, electronic configuration suffered by diverse differences from the pristine ZnIn2S4 NSs [150]. So, the DFT-based calculations showed that O2-doping effectively reinforces DOS at VBM versus pristine ZnIn2S4, enlightening creation of enhanced charge density around VBM. Both of CB and VB edge in O-doped ZnIn2S4 showed the upshift concerning ZnIn2S4 NSs, as verified through UV–Vis absorption spectrum and XPS VB spectrum. It enhanced CBM as well as improved the VB distance across obtained with very superior mobility and improved the expenditure of photo-generated h+s, thus to support H2 production. Wang and co-authors studied that C-atoms doping can efficiently refrain electronic configuration of h-BN (Fig. 11) [151]. So, the DFT simulation showed that BG of h-BN was obtained to be 4.56 eV. After C-doping in structure, BG was notably reduced. Regarding B11C12N9 compound, BG was narrowed to 2.00 eV, through VB and CB edges which mostly consist of C2p orbitals. B11C12N9 VB top states were not localized in comparison with pure BN. Taking advantage of C-doping with sp2-delocalization system, ultra-thin C-BN NSs with thickness of almost 3–4 nm were achieved and displayed an outstanding vis-light photo-catalytic performance to evolution of H2 and reduction of CO2. Considering insulator characteristic of pristine h-BN, it is illustrated that nonmetal doping can endorse photo-catalytic performance and also create promising photo-catalytic performance.

Adapted with permission from Ref. [151]
a HR-TEM of BCN-30. b Dark-field TEM image of BCN-30 and element map. c HR-STEM of the BCN-30 sample along (002) facet. d XRD of h-BCN. g NMR spectra of BCN-30. e Raman spectrum of h-BN and BCN-30 and physical mixture of h-BN and graphite. h EELS spectrum of BCN-x. f UV–Vis diffuse reflectance spectra of BCN-x. i BG calculation of BCN-x from the (F(R) E)n versus E plots.
4.4 Defect Engineering
Aside from doping, defect engineering also demonstrates an important effect on ultra-thin 2DMs, in case of photo-catalysis. Owing to 2D atomic size thin structure, in the presence of defects, it has strong influence on fundamental properties, in spite of a very low-level doping. In relation to huge surface defects formation in the bulk materials, ultra-thin 2DMsNSs with relatively small atomic escape energy can propose an important chance to get a range of defects. So, it is necessary to construct surface defects, for instance anion, cation vacancies, pits, vacancy association, and distortions, to efficiently optimize electronic configuration of ultra-thin 2D photo-catalysts.
4.4.1 Anion Vacancies
In anion vacancy type, VO was broadly studied due to its small creation energy and prevalence in the oxide materials [152]. For example, Fengcai Lei et al. [84] studied that by fast heating of intermediate In(OH)3 NSs in O2 or air, Vo-rich and Vo-deficient In2O3 NSs were formed in fully controlled way, respectively. Figure 12 shows the AFM image, which showed thickness of In2O3 NSs to be almost 0.9 nm, enlightening controlled formation of In2O3 materials with atomic thickness. ESR and XPS spectrum results showed the presence of Vo. The observed 531.4 eV peak showed Vo-rich ultra-thin In2O3 NSs, which have maximum peak area, signifying that more VO-rich ultra-thin In2O3 NSs were formed compared to Vo-poor ultra-thin In2O3 NSs and bulk counterpart. Moreover, sharp Vo signal at g = 2.004 in ESR spectrum was also observed that shows Vo-rich In2O3 NS sample holds the highest level of Vo. In Vo engineering, electronic configuration of In2O3 NSs with rich Vo will experience noticeable change. As authorized through DRS analysis and the XPS VB, spectrum, Vo-rich In2O3 NSs showed a narrowed BG and upshifted VB edge. DFT simulations clearly show that enhanced DOS at VBM was created and a novel defect concentration showed Vo-rich In2O3 than Vo-poor In2O3 NSs. So, the Vo-rich In2O3 NSs acquired higher carrier level and enhanced electric field in space charge regions. The e−s were further simply excited into CB in irradiation. Therefore, Vo-rich In2O3 NSs showed 2.5 and 15 times enhanced photo-catalytic performance as compared to Vo-poor In2O3 NSs as well as bulk In2O3, correspondingly, for H2O oxidation. Such outcomes certainly verified the efficient role of anion vacancy in electronic configuration engineering.

Adapted with permission from Ref. [84]
a Scheme showing the development of VO-rich/VO-poor atomically thin In2O3 porous NSs under special environment. b Time-dependent small-angle XRD patterns for the as-synthesized precursors. c–f Characterizations for the VO-rich atomically thin In2O3 porous NSs obtained via rapid thermal heat treatment of In(OH)3 NSs in air. c, d TEM/HR-TEM image. e AFM analysis. f O 1s XPS spectra. g Electron spins resonance spectrum. h Simulated DOS of O2 defect five-atom-thickness In2O3 slab. i ideal five-atom-thickness In2O3 slab.
4.4.2 Cation Vacancies
Besides anion, cation vacancies are extremely efficient approach to cause useful electronic structure modification of ultra-thin nano-structure due to multifarious electron arrangement and orbit. For example, vanadium (V) vacancies (Vv) were initiated in single-unit-cell BiVO4 NSs (1.28 nm) along diverse quantities (Fig. 13) [153]. The atomic level concentration of the Vv was done through positron annihilation spectrometry (PAS) and X-ray fluorescence (XRF). For BiVO4 the shortest life component (τ1, around 200 ps) approved from PAS that trapped at Vv, helpful for subsistence of Vv for two samples. Comparative positron intensity duration for Vv-rich BiVO4 NSs was denoted for higher Vv level. Furthermore, elemental ratio of t V and Bi was verified to be 0.914 and 0.976 in Vv-rich BiVO4 NSs and Vv-poor BiVO4 NSs, in that order proposing concentration difference of Vv. Benefiting from incidence of Vv, a novel defect level can be produced in BG of BiVO4, as confirmed via DFT simulation, which leads e−s further capable to be excited into C.B. Additionally, higher DOS at VB edge was achieved because of Vv engineering. So, engineered vacancies increased the light harvest and promoted electronic conductivity for Vv-rich BiVO4. Simultaneously, abundant Vv allowed an efficient charge separation that prolonged carriers’ lifetime from 74.5 to 143.6 ns. Taking advantages from such Vv, an advanced photo-catalytic performance was attained from methanol synthesis rate up to 398.3 µmol g−1 h−1.

Adapted with permission from Ref. [154]
a, b TEM and AFM analysis of one-unit-cell thickness Vv-rich o-BiVO4. C–e Defects study of Vv-rich and Vv-poor o-BiVO4 atomic layers. c Positron duration spectra. d, e Scheme of entrapped positrons. f, g DOSs calculation of the V defects in o-BiVO4 single-unit-cell layer slab and pure o-BiVO4 single-unit-cell layer slab (f), along [001] direction (g).
4.4.3 Associated Vacancies
The lost surface atoms not just introduced mono-vacancy but also vacancies associated were able to appear. As a result of multi-atomic vacancy coupling, vacancy associated can strongly engineer electronic structure and cause amazing electronic performance. For instance, triple vacancy of VBi″′VO··VBi″′ were built in ultra-thin BiOCl NSs (thickness = 2.7 nm) with dimension engineering (Fig. 14) [119]. Generated associated triple vacancy VBi″′VO··VBi″′ was verified through PAS. When Bi-atoms’ outer surface was exposed in BiOCl crystal configuration, it is very likely to break out from lattice to make vacancy. While thickness was decreased to an atomic size, O2-atoms that linked to Bi-atoms in an internal layer also escaped more effortlessly. So, control defects in BiOCl nano-plates were separated VBi″′, whereas that changed its associated vacancy VBi″′VO··VBi″′ in ultra-thin BiOCl NSs. Different defect types certainly affect the electronic structure that guarantees BiOCl NSs with enhanced adsorption of RhB molecules due to further negative charge. Benefiting from defect types changing from VBi″′ to VBi″′VO··VBi″′, ultra-thin BiOCl NSs showed both upshifted CB and VB potentials that favor charge mobility and therefore allowed enhanced separation of (e−–h+)-pairs. Therefore, ultra-thin BiOCl NSs showed great solar photo-catalytic activity toward removal of pollutants.

Adapted with permission from Ref. [119]
Scheme showing the crystal structure of BiOCl. a 3D-projection. b, c [Bi2O2]2+ layers along with the [010] and [001] directions, correspondingly. d TEM/HR-TEM of BiOCl NSs. e Positron lifetime spectra of ultra-thin BiOCl NSs and BiOCl NPs. f, g Scheme showing trapped positrons of VBi″′ defect and VBi″′VO··VBi″′-associated vacancy, correspondingly.
4.4.4 Distortion
Besides the vacancies, other defects, for instance, distortions and pits were also sufficient policies for changing electronic structure of the ultra-thin 2D photo-catalysts. For instance, O’Hare and his research team [99] formed NiTi-LDH NSs through controlled thickness during reverse micro-emulsion approach. As obtained NiTi-LDH NSs, local atomic arrangement was examined through the X-ray absorption near-edge spectroscopy (XANES). While thickness significantly decreased, the titanium cation (Ti4+) with lowered oxidation state was achieved in NiTi-LDH NSs (≈ 2 nm thickness), whereas it is approximately completely Ti4+ in bulk NiTi-LDH. In comparison with bulk NiTi-LDH, lower coordination numbers of Ti-cations were seeing in NiTi-LDH NSs and experience severs crystal deformations. Consequently, NiTi-LDH NSs showed distinguishing electronic crystal lattice and enhanced e− transfer effectiveness comparative to bulk NiTi-LDH.
4.4.5 Pits
Pits formation gives a good technique to enhance surface defects which produced more coordinated unsaturated atoms with dangling bonds around pits in NSs. Through thermal treatment of bulk g-C3N4 inNH3 environment, NSs are efficiently exfoliated and many in-plane pits were built in g-C3N4 NSs [124]. In-plane pits formation damaged g-C3N4 plane structure and supplies promising active sites with dangling bonds. These dangling bonds serve as cross-plane diffusion pathways to speed up mass transfer and charge diffusion. Furthermore, formed pits are promoted as a result in creation of C-vacancies owing to unbalance structure. So, g-C3N4 NSs showed improved BG and absolute light absorption area matching with bulk counterpart. Simultaneously, pit-rich g-C3N4 NSs guarantee superior CB and VB potential and give advanced e− donor density. Thus, 20 times high photo-catalytic H2-evolution activity was attained in vis-light irradiation. The aforementioned investigation results give a new and deep insight to comprehend method of action of surface defects in supporting photo-catalytic activity from energy band structure, surface charge, and SASs. Usually, as relative to defect poor counterpart, engineered defects in ultra-thin 2DMs can alter electronic configuration, generally with improved DOS at edge of VB or CB, or even create a novel defect concentration in between forbidden band. Generally, light absorption possibility of genuine materials with redshift and hence light harvesting capability can be improved. At surface, limited charge density can also be altered and might make possible adsorption and activation of the target molecules. Simultaneously, formed surface defects can develop carrier level in the photo-catalysts and provide charge separation centers to entrap the carriers, encouraging consumption effectiveness of h+s and e−s for analogous interfacial redox responses.
4.5 Anisotropic Effects in Catalysis
Photo-catalytic routes could show vital to sustainable manufacturing of fuels and chemicals necessary for carbon–neutral society. An (e−–h+) recombination is a serious issue that has till date limited efficiency majority of potential photo-catalysts. Therefore, Matteo Cargnello et al. [155] showed the efficiency of anisotropy in enhancing charge separation and thus increasing activity of TiO2 photo-catalytic method. Particularly, they showed that H2 fabrication in homogeneous, 1D brookite-TiO2 nano-rods was highly improved through engineering their length. Utilizing respective characterization techniques to separately investigate excited e−s and h+s, linked high observed reaction rates to anisotropic arrangement support competent carrier use. The QY for H2-fabrication from C2H5OH, C3H8O3, and C6H12O6 as high as 65%, 35%, and 6%, correspondingly, showed generalization of this method for enhancing the photo-activity of SC-NMs for a broad range of reaction systems.
5 Hybridization
Unlike bulk materials, 2DMs have become a hot topic in academic field due to their atomic layer thickness, broadband absorption, and ultrafast optical response, which have been widely applied in the ultrafast laser generation [156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183], optical switching and modulators [184,185,186,187,188,189,190,191,192,193,194,195,196,197], optoelectronics devices [183, 198,199,200,201,202,203], and biosensor and biotherapy [14, 44, 204,205,206,207,208,209,210,211,212,213,214]. 2DMs have an ultra-large SSA to make sure that surface state is even more significant compared to bulk inside. Photo-generated charge carriers will be distributed at surface to engage in the redox reactions, to be discussed next. So, surface hybridization to present size element to support consumption effectiveness of charge carriers is enviable below precondition of 2D arrangement. Consistent with surface hybridization, numerous representative hybridizations with 2D structure are introduced, e.g., QDs/2DMs, single atoms/2DMs, molecular/2DMs, and 2D–2D stacked materials.
5.1 Quantum Dots/2DMs Hybridization
Coordinated unsaturated surface atoms of NPs have dangling bonds that used energy. To further decrease NPs’ size, bigger part of surface atoms compared with total atoms will be attained and their regular atom binding energy must be high. So, if the size of NPs can control the QDs and modify 2DMs, interfacial strong coupling among them can be manufactured. Furthermore, QDs can show high dispersion on 2DMs because of their small size; those probably effective co-catalysts furthermore enhance the photo-catalytic activity. To develop operation effectiveness of Ag, Ag-QDs with size > 5 nm were formed. After hybridization with BiOBr NSs, photo-catalytic activity has been significantly boosted to degrade tetracycline hydrochloride, ciprofloxacin (CIP), and rhodamine B after vis-light irradiation. It was studied that tailored Ag-QDs activate molecular O2 through hot e− which decrease after vis-light exposure. The Ag-QDs can concurrently provide charge separation centers, adsorption centers, and photo-catalytic reaction centers, which are dependable on enhanced photo-catalytic efficiency. To decrease service of noble metals, non-noble metals or even metals-free QDs are taken as other choice. Also, thickness of subjected 2DMs can be further decreased to SL, in order to get improved dispersion and interfacial contact. For example, nitrogen-doped carbon QDs (N-CQDs) through 3 nm size were formed by facile hydrothermal method and later modified at BiOI NSs’ atomically thin surface [120]. The AFM analysis showed that average thickness of BiOI was almost 0.9 nm, proposing SL arrangement.
After N-CQDs, the N-CQDs/BiOI matters introduction displayed significantly extended lifetime of photo-generated charge carriers, as showed through time-resolved transient photo-luminescence (PL) decay and immediate photo-current. BiOI atomic-level configuration makes sure prominently quick bulk charge diffusion to surface and conjugated π-modified N-CQDs configuration, which effectively endorse surface charge separation, resulting in longer carrier duration. Therefore, the photo-catalytic activity and the N-CQDs/BiOI materials active species concentration enhanced considerably. Kang and co-authors also confirmed that C-QDs can work as chemical catalyst to really improve the photo-catalytic H2O splitting via C3N4 (Fig. 15) [215]. Different from traditional single step 4e− reaction from H2O splitting, 2e−/2e− two-step path was followed by the C-QDs-C3N4, where C3N4 is added to transfer H2O to H2 and H2O2. Moreover, C-QDs are accountable in H2O2 decomposition and O2 evolution. Therefore, outstanding photo-catalytic H2O splitting effectiveness can be obtained, with 2.0% solar to H2 efficiency and robust stability in 200 recycle run after 200 days. Similarly, there are also some other systems concerning QD/2DMs hybridization to further enhance photo-catalytic efficiency, for example CdSe QDs, Zn–Ag–In–S QDs, NiS2 QDs, and so on [216]. Such findings certainly verified superiority of QD modification, and QD/2D arrangement might be efficient substitute for configuration to get improved photo-catalytic performance.

Adapted with permission from Ref. [215]
a–d TEM of C-QDs/C3N4. e UV–Vis absorption spectrum of C3N4 and C-QDs/C3N4 photo-catalysts. f Time period of O2 and H2 production from H2O after vis-light irradiation catalyzed via C-QDs/C3N4. g Wavelength-based QE (red dots) of H2O splitting via C-QDs/C3N4.
5.2 Single Atoms/2DMs Hybridization
To further enhance photo-catalytic activity, size reduction of NPs to single atoms is possibly a probable plan. However, fraction of unsaturated coordination bonds of monoatom maximizes and enables a strong surface effect [217]. The first studied monoatom-based catalysis by Zhang et al. [218] became an interested topic in photo-catalysis direction. The monoatom-based photo-catalyst was based on isolated single atom on support materials surface, in the form of dispersion or coordination. Monoatoms-based strategy can improve photo-catalytic activity and offer another technique to alter the selectivity. As well, active single atom, chemical bonding in metal single atom, and 2DM NSs-based supports have become a strong and simpler charge transfer method. So, it is very attractive to create a single-atom/2DMs hybridization to obtain a high photo-catalytic activity [219]. Wu and co-workers [220] studied single Pt-atom as co-catalysts to advance photo-catalytic H2 evolution activity of C3N4 NSs. A simple liquid phase reaction with C3N4 and H2PtCl6-coupled low-temperature annealing was applied to form Pt-single atoms/C3N4. High-angle annular dark-field STEM (HAADF-STEM) was utilized to establish allocation and arrangement of Pt. The individual clear spots matching to Pt-atoms were viewed to be consistently dispersed on g-C3N4, with ~ 99.4% Pt size of > 0.2 nm, showing a Pt subsist completely as monoatoms. On the other hand, when the Pt loading quantity reaches 0.38%, the Pt-atoms dispersion was denser and formed numerous sub-nanometer clusters. Extended X-ray absorption fine structure (EXAFS) spectroscopy was utilized to investigate local atomic configuration of the Pt/C3N4. The Pt-atoms coordination number was about 5, through bond distance of ~ 2.03 Å, exposing those Pt-atoms that were dispersed on the top of C3N4 system. Following production of single Pt-atom/C3N4 arrangement, photo-catalytic H2-evolution activity was significantly enhanced. The H2-evolution rate of Pt/C3N4 (0.16 wt% Pt loading) achieved was almost 318 µmol h−1, about 50 times advanced, as compared to pristine-C3N4. Simultaneously, single Pt-atom/C3N4 showed admirable stability for H2-evolution and isolated single Pt-atom still stays at C3N4 after circulations. The desirable quality of UTAS, surface trap states of C3N4 verified was basically changed because of isolated single Pt-atom that extends the carrier duration and provides more chances for e−s to engage in H+-reduction method. Moreover, separated single Rh-atoms were spread on 2D-TiO2 NSs along with homogeneous 0.7 nm thickness via calcination, protonation, and coupled exfoliation method [221]. In HAADF-STEM image, separated brightest spots were observed that showed Rh-atoms but intermediate brightness spots symbolized Ti-atoms. The EXAFS showed that Rh species in single Rh-atoms/TiO2 displayed an analogous chemical setting as Rh2O3 showed bonding to O-atoms which was hence oxidized. Mono-Rh-atom co-catalysts were served as a reaction core for photo-catalytic-based H2-evolution, consistent with DFT simulations. So, H2-evolution rate was boosted 10 times as compared to pure TiO2 NSs. Although single atoms were engaged in catalysis, there were also existed numerous matters to be determined. Normally, the sustained content of monoatom was comparatively small and noble metal single-atom showed main types. It is very attractive to boost quantity of supported metal atoms with isolated single-atom arrangement and broaden it to other non-noble metals. Moreover, it is required to promote the stability, in order to meet potential industrial uses. The facts that single isolated metal atoms had high surface energy were shown, in which isolated metal atoms strongly cooperate with support surfaces. Through influenced metal atoms interactions with surface defects (elevated energy sites) on support, hybridization energy scheme might turn into a local minimum. Consequently, mono-metal atoms can be fastened as well as kept stable. Particularly in ultra-thin 2DMs, surface defects are liable to be generated because of ultra-large SSA and minute atomic flee energy. So, it is possible to construct monoatom anchored surface DR ultra-thin 2D arrangement to elevate photo-catalytic activity.
5.3 Molecular/2DMs Hybridization
Despite single isolated atoms, single molecule materials can also be used to engineer electronic structure by acting as a co-catalyst to enhance photo-catalytic activity [222]. Profiting from sub-nano-pores in C3N4, molecular TiO2 was included into C3N4 NSs by facile polycondensation of precursors with dicyandiamide and TiO2-ions [223]. The morphology of clean ultra-thin TiO-C3N4 NSs was obtained and confirmed by TEM analysis, with thickness of about 3−3.3 nm. From HAADF-STEM analysis and elemental mapping, TiO2 was originated to be consistently dispersed on C3N4 framework with isolated format. These results suggested that molecular TiO2 was effectively built in C3N4 framework equally. Local Ti–O geometrical and electronic structures in sub-nano-pores of C3N4 NSs were found through XAFS. Usually, anatase-TiO2 displayed well-defined triple pre-edge characteristics that can be recognized to distort TiO6 pattern with six coordinated O2-atoms. Dissimilar from TiO2 result, Ti–O in C3N4 exhibited a single pre-edge feature with non-symmetric structure. The EXAFS showed that Ti-atoms were located in C3N4 h+s that was coordinated with six N2-atoms in C3N4 and one O2 atom out of plane in C3N4. Benefiting from TiO2 molecule insertion, TiO-C3N4 NSs displayed a narrowed BG in comparison with pristine C3N4 with a reduction in CB position. It is resultant from more electron involvement of Ti–O into C3N4 and enhances π–e− delocalization in conjugated structure. TiO-C3N4-engineered electronic structure can also enhance charge carrier separation. Therefore, TiO-C3N4 enhanced the photo-catalytic performance for ·OH generation and removal of pollutant. In photo-catalytic method, h+s transfer slower kinetics which brings massive carrier recombination in Achilles’ heel of photo-catalytic conversion activity. Even though ultra-thin 2D-structure permits quick charge migration in bulk phase, lack of surface charge separation centers will also spoil overall photo-catalytic activity. Utilizing strategy to encourage surface charge separation, particularly h+s transfer is extremely required. Instead, it looks feasible to utilize H2O soluble molecular materials as homogeneous co-catalyst and hence also optimize photo-catalytic performance. Wu and his research team [220] formed H2O-soluble molecular trifluoroacetic acid (TFA) as co-catalysts to enhance photo-catalytic H2-evolution performance of K4Nb6O17 NSs. Taking advantage of reversible redox couple TFA·/TFA− as well as high active intermolecular radical responses, TFA molecule served as strong h+s-shuttle, allowing efficient move of the photo-generated h+s and ensuing elevated charge separation effectiveness. The TFA increment enhanced H2-evolution rate regularly and the maximum rate reached 6344 µmol g−1 h−1, when TFA/K4Nb6O17 molar ratio was 25.6. This optimum H2 yielding rate was ~ 32 times higher compared to pristine K4Nb6O17 NSs, certainly signifying this molecular co-catalyst. As solid-state co-catalysts, they are restricted from limited contact areas in co-catalysts and host photo-catalyst, and surface charge separation cannot be completely definite. When overmuch solid-state co-catalysts were anchored on photo-catalyst, the SASs will be covered that deficient in the satisfactory active sites easy to get reactant molecules. As a result, H2O-soluble molecular materials such as molecular co-catalysts can equally disperse in the solution and give greatest available area to host photo-catalysts. So, developed molecular co-catalyst approaches perhaps are a possible way in a competent separation of photo-generated carriers and therefore improve photo-catalytic performance.
5.4 2D–2D Stacking Materials Hybridization
While constructing 2D–2D stacks, it is a largely applied method to boost photo-catalytic efficiency. Particularly for layered materials, lattice mismatch was reduced due to comparable layered structures, and 2D–2D stacking with close associates was formed. For example, Zhang and co-workers [139] studied SL Bi12O17Cl2 with surface Vo via Li-intercalation-based exfoliation approach. Afterward, SLs MoS2 NSs were assembled onto SL-Bi12O17Cl2 through surface Vv and build Bi-S bonds in Bi12O17Cl2 and MoS2. TEM image well matched with an elemental mapping results proposed that numerous tiny MoS2 NSs were strongly anchored on a large Bi12O17Cl2 NS to make 2D stacking hetero-structure (Fig. 16); normal thickness of large size and small size NSs was ~ 0.717 and 0.686 nm that concurred with Bi12O17Cl2 and MoS2 SLs, in that order.

Adapted with permission from Ref. [224]
a TEM top view analysis. b–e elemental mapping images, f XPS spectrum. h, i AFM analysis. m TEM images side view. n Atomic resolution HAADF-STEM image. o–s Respective EELS elemental maps of BOC-MS. g S K-edge XANES spectrum of BOC-MS, 1L-MS, and bulk MS. j Height profiles along lines in i. k Comparison of 1L-BOC and 1L-MS in BOC-MS average thicknesses. Error bars in (k) show s.d. over 100 self-sufficient AFM calculations. SLs MoS2 and Bi12O17Cl2 theoretical thicknesses.
The atomic resolution HAADF-STEM analysis and resultant EELS elemental mapping showed that it was obviously studied and SLs-MoS2 were anchored selectively on (Bi12O17) end faces to construct (Cl2)–(Bi12O17)–(MoS2). As charge density surrounding (Bi12O17)2+ layer was superior compared to (Cl2)2− layer, photo-generated e−s and h+s were ambitious to (Bi12O17)2+ and (Cl2)+ end faces under irradiation, correspondingly. Photo-generated e–s flowed in between MoS2 SLs through formed Bi-S bonds and enable efficient charge separation (ultra-long duration of carrier 3446 ns), as proofed through TA spectroscopy. Taking advantage of atomic size thickness, efficient directed interface charge separation, and plentiful H2-evolution sites in MoS2, acquired MoS2/Bi12O17Cl2 bilayers displayed great vis-light photo-catalytic H2-evolution performance. Using ascorbic acid as h+s sacrificial agent, H2-evolution rate can turn up 33 mmol h−1 g−1 and quantum effectiveness of 36% at 420 nm. Other than MoS2/Bi12O17Cl2, there are several investigations about 2D stacking to optimize photo-catalytic efficiency, for instance NiO/Ca2Nb3O10 [225], MoS2/TiO2 [224], MoS2/CdS [226], WS2/CdS [226], MoS2/C3N4 [227], SnS2/C3N4 [228], Fe2O3/C3N4 [229], C3N4/Bi4O5I2, ZnCr-LDH/layered titanate [230], and ZnIn2S4/MoSe2. For example, a distinctive 2D stacking structure has more benefits. It increased the available area about planar interface in 2D/2D structures and reduced barriers for e− transportation via co-catalyst, and therefore promoted the interfacial charge transfer development through e− tunneling effect. Furthermore, these 2D thin layers can ease light blocking effect of co-catalyst; therefore, sufficient light can contact the host photo-catalyst. It is required to alter 2D-components and reinforce interfacial acting force to promote highly efficient 2D/2D photo-catalysts.
6 Photo-catalytic Applications
Based on the aforementioned consequences, ultra-thin 2DMs displayed huge benefits for the photo-catalysis from microstructure, BG, electronic configuration, and surface nature. Thus far, advanced ultra-thin 2DMs NSs were functional as photo-catalysts for diverse photo-catalytic uses. So, development of flexible photo-catalytic uses by 2DMs for H2O oxidation, H2-evolution, CO2 reduction, N2-fixation, organic synthesis, and pollutants removal will be explained in detail in the next section.
6.1 Water (H2O) Oxidation
Hydrogen (H2) that has the highest energy density is measured as one of the potential energy carriers for storing solar energy in chemical bond energy form between two H atoms. In between different methods for conversion of sunlight and H2O into H2, photoelectrochemical (PEC) H2O splitting using SC photo-electrodes has gained most interest due to three main benefits:
-
1.
Production of H2 and O2 at respective electrodes that eradicate separation problems.
-
2.
Operation potential under environmental conditions.
-
3.
Potential for manufacturing of a system which consists of just stable and copious inorganic materials.
Designing vis-light-active SCs for H2O splitting needs an appropriate BG and band position, efficient charge separation, fast charge movement, and longtime durability in aqueous solutions. An attractive design approach to fulfill these requirements is to merge 2D materials (e.g., graphene, MoS2, g-C3N4) with appropriate SCs. It is usually known that H2 is very potential green fuels with benefits, for example high specific energy, multiple use approaches, and pollution-free combustion product. It displayed magnificent view in prospect of sustainable energy use, if it is created through sustainable skill. The photo-catalytic H2O splitting into H2 and O2 is viewed as Holy Grail infield of chemistry through just sustainable solar light as energy input, photo-catalysts as medium, and H2O as reaction source. As major forward step was accomplished, effectiveness of H2O splitting is still restricted in majority of photo-catalytic methods. Usually, H2O-oxidation is efficiency, limited method in photo-catalytic H2O splitting schemes because of complex four h+s complex redox method. So, it is highly in demand and imperative to propose photo-catalyst with robust solar H2O-oxidation method. Recent studies illustrated that 2DMs-based photo-catalysts are very capable choice for solar H2O-oxidation [231, 232]. The freestanding SLs ZnSe with four-atomic thickness was formed through ultrasonic exfoliation from lamellar hybrid intermediate (Zn2Se2)(pa) (pa represents n-propylamine) [231]. The U-XAFS, local atomic structures, and electronic configurations of the ZnSe SLs were studied. For Zn K-edge kχ(k) oscillation curves as shown in (Fig. 17a), the ZnSe SLs displayed clear distinction comparative to ZnSe-pa SLs and bulk ZnSe, showing significant variation of local atomic arrangement. The R-space curves of ZnSe samples showed that peaks positioned at 2.11 and 3.63 Å were attributed to the nearest Zn–Se and next nearest Zn–Zn coordination in bulk ZnSe (Fig. 17b). While ZnSe size was decreased to an atomic level, local atomic configuration experiences outstanding changes. ZnSe peak was shifted to 2.17 Å, and next the nearest Zn–Zn distances (3.85 Å) were decreased. Simultaneously, Se–Se distances in SLs ZnSe NSs were extended from 4.012 to 4.11 Å of bulk ZnSe. Such findings certainly showed reality of surface distortion in SL structure that reduced surface energy and allowed exceptional stability of ZnSe SLs. Moreover, surface deformation of SL ZnSe will consequently be in the form of enhanced DOSs at CB edge that might further make sure a high charge carrier transfer rate (Fig. 17c).

Adapted with permission from Ref. [233]
a Se and Zn K-edge extended XAFS oscillation function kχ(k). b Equivalent Fourier transforms; red, blue, and black lines show ZnSe SLs, ZnSe-pa SLs, and bulk ZnSe, in that order. c Simulated DOS; black, blue, and olive lines show total, Se sp, and Zn sp state densities, correspondingly. d Photo-current density versus utilized potential curves in chopped 300 W Xe-lamp irradiation.
Taking advantages from SL structure with surface defects, ZnSe SLs show strong light absorption, enhanced charge separation effectiveness, and small charge transfer resistance. Consequently, SLs ZnSe NSs exhibited 195 times superior photo-catalytic performance compared to bulk ZnSe for H2O oxidation after Xe-lamp irradiation (Fig. 18d). As H2O oxidation to evolve O2 is completely h+-contributed reaction, enhancing h+-use rate perhaps is an efficient plan to enhance photo-catalytic H2O-oxidation. Liu et al. [136] created several pore constructions in ultra-thin WO3 NSs through fast-heating approach on earlier exfoliated WO3·2H2O NSs. As migration direction of the photo-generated h+s was along {001} [2,3,4, 16,17,18,19,20,21,22,23,24,25,26] facets in the x-direction in W–O–W chains on WO3 NSs, with long itinerant pathway the photo-generated h+s certainly undergo several recombinations of charge carriers, which acutely prevent photo-catalytic performance. The created pores efficiently shorten diffusion way of h+s and conduce to H2O oxidation to make O2 at WO3 surface. Additionally, plentiful dangling bond along pore environment created good circumstances to make easy chemisorptions of molecular reaction that finally boosted O2-evolution kinetics. As a result, 18 times superior photo-catalytic H2O oxidation was attained for pore-rich WO3 ultra-thin NSs comparative to bulk WO3. It shows significant strategy to promote conversion efficiency by using ultra-thin 2D configuration to the photo-catalytic H2O oxidation. Additionally, many other photo-catalysts with ultra-thin 2D-structure can also show an exceptional photo-catalytic behavior toward H2O oxidation, for instance SnS2 [234], SnSe [235], SnS, Fe2O3 [236], NiTi-LDH, and also engineered materials like In2O3 with Vo engineered [84], Co-doped In2S3 [145], pit-rich BiOCl [237], and so on.

Adapted with permission from Ref. [238]
a Photographs of WS2 films on glass with different thicknesses. b UV–Vis absorbance of WS2 different thin films. c PEC performance illustrated as J–V polarization curves for WS2 different films size. d IPCE measurements of WS2 different thin films size on p-Si photo-cathodes.
6.2 H2-Production
As innovative investigation for photo-catalytic H2-evolution based on TiO2 in 1972, photo-catalytic H2-evolution from H2O was at front position of chemistry research to solve worldwide energy problem. Many amazing catalytic materials were used in photo-catalytic H2-production; majority are still said to have quite small photo-catalytic effectiveness, which could not meet the necessities of realistic uses on large industrial scale. Current research for the 2DMs established that rising 2D-SCs with suitable energy BG were a talented choice to get outstanding H2-evolution performance [239]. In case of abundant SCs, the Cu2O with unique CB, positioned at ~ 0.7 V negative as compared to H2-evolution potential, is possibly a competent catalyst in H2-conversion from solar. To realize elevated H2-evolution efficiency, atomic-sized 2D-NSs, e.g., cubic Cu2O, were formed. The AFM study showed thickness of about 0.62 nm, related to four-atomic-level thickness of Cu2O in [01-1] direction. Therefore, surface energy of the cubic Cu2O was conformed to the order of (111) < (100) < (110). As (110) and (01-1) surfaces are equivalent facets (01-1), facet in atomic-level thin Cu2O NSs also showed a great surface energy that brings a good activity. From considerably reduced thickness, atomically thin Cu2O NSs’ electronic structure was distinct from bulk equivalent. Utilizing DFT simulations, an atomically thin Cu2O NSs showed really enhanced DOSs at edge of VB as compared to bulk Cu2O. At similar time, extended CB edge was also studied in 2D Cu2O NSs as compared to bulk Cu2O, enlightening that atomic-level thick Cu2O has high carrier mobility and small BG. Profiting from such advantages, above 36 times higher photo-catalytic H2-evolution rate was reached from 2D Cu2O NSs after vis-light irradiation. These results certainly illustrated that 2DMs can bring huge advantage for H2-evolution as well as a series of extraordinary activities. In addition to the advancement in H2-evolution, more modification in ultra-thin 2DMs structures was required. For instance, through doping O2 into ZnIn2S4 NSs, H2-evolution rate of O-doped ZnIn2S4 can attain 2120 µmol h−1 g−1 from aqueous solution containing 0.25 m Na2SO3 and 0.35 m Na2S after visible light illumination lacking any co-catalyst that was 4.5 times high as compared to pure ZnIn2S4 [150].
Local atomic structures of formed materials were studied by XAFS. The O2 doping in ZnIn2S4 NSs created high structure distortion through substitution of O2-atoms for sulfur. The engineered local atomic and electronic structure will experience observable deviation. The DFT simulation showed that O2 doping can boost DOS at VBM with respect to pure ZnIn2S4, signifying creation of enhanced charge density about VBM. XPS results showed the valence spectra and estimated BG, where CBM and VBM of O-doped ZnIn2S4 ultra-thin NSs show upshifting in comparison with pure ZnIn2S4. So, average recovery duration of carriers for O-doped ZnIn2S4 NSs was about 1.53 factors long-lasting relative to pure ZnIn2S4, attaining 110 ps. Hence, these advantages showed that O-doped ZnIn2S4 can show really enhanced photo-catalytic activity for H2-evolution. These findings showed that doping was an efficient policy to alter local electronic and atomic structure of 2DMs that can influence charge separation or migration and at the end optimizes H2-evolution.
Moreover, though considering major ultra-thin 2D-SCs NS materials lacks satisfactory H2-evolving sites, it is necessary to establish plentiful H2-evolution positions, for example, isolated Pt-atom or FL-TMDCs to more improve H2-evolution performance. Therefore, Wang and co-authors [227] studied FL MoS2 to C3N4 NSs to improve photo-catalytic H2-evolution. In the formation method, C3N4 was absorbed in (NH4)2MoS4 H2O solution and after that sulfidation was done through H2S gas, at 350 °C. C3N4 and MoS2 have equivalent layer formation, which can reduce lattice mismatch and help planar development of MoS2 slabs after using C3N4 surface. Consequently, an inorganic–organic 2D–2D stacking was formed using G-like thin-layer hetero-junctions. Plentiful H2-evolution sites were formed via FL MoS2 NSs. Moreover, dispersed MoS2 thin layers on C3N4 NSs surface can provide the superior effectiveness compared to multilayer MoS2 because of e-tunneling effect via MoS2 thin layers from reaction interfaces. On the basis of support charge separation and plentiful H2-evolving sites persuaded through FL MoS2 NSs, acquired MoS2/C3N4 2D junctions exhibited better photo-catalytic H2-evolution activity in comparison with pristine C3N4. The 0.2 wt% MoS2 is the best sample that illustrated the highest H2-evolving rate, with an obvious 2.1% QY recorded at 420 nm. Moreover, WS2 can be utilized as a catalyst in WS2/p-type Si photo-cathode hetero-junctions. Kwon et al. [238] examined WS2/p-Si photo-cathode for photo-catalytic based HER. Figure 18a shows the as-prepared WS2 thin film color changes from yellow to brown with increasing thickness, whereas absorbance of film regularly enhances. However, absorption peak position almost remains constant (Fig. 18b). The PEC demonstrated that photo-catalytic HER performance for 23 nm WS2/p-Si showed the maximum current density of 8.375 mA cm−2 at 0 V and 72% incident photon to current conversion efficiency (IPCE) (Fig. 18c, d). So, it shows that merger of TMDs (WS2, MoS2), along traditional SCs, for example Si, is capable for efficient PEC H2O splitting.
Similarly, Wang et al. [240] studied physicochemical nature of Ti3C2Tx MXene coupled with TiO2 for photo-catalytic HER (Fig. 19a) [241]. The 5 wt% content of Ti3C2Tx in TiO2/Ti3C2Tx nano-composite showed a 400% augmentation for the photo-catalytic HER than rutile TiO2. Therefore, Ti3C2Tx gives a 2D-podium to interrelate with consistently fabricated TiO2 to make possible partition of photo-generated (e−–h+)-pairs to slow charge recombination. Fascinatingly, TiO2 NPs were well dispersed at Ti3C2Tx surface without harsh aggregation. Additionally, Ti3C2Tx, Nb2CTx, and Ti2CTx were also utilized as reducing co-catalysts after reacting with TiO2 and increase photo-activity in the HER [240]. Fascinatingly, Nb2CTx and Ti2CTx show better HER catalytic activity as compared to Ti3C2Tx, while coupling with TiO2 as co-catalysts (Fig. 19b). It can be dragged through Schottky barrier (SB) and work function of every MXene, where Nb2CTx shows the maximum work function (~ 4.1 eV) for direct proof of the highest HER activity. In reaction system, TiO2 surface was excited after irradiation with light to generate e−s and h+s. As a result of different Fermi levels of MXene and TiO2, photo-generated e+s was transferred from CB of TiO2 to MXene. Furthermore, adding positive charges in TiO2 and negative charges in MXene, CB and VB were turned upward (Fig. 19c), leading to form SB at TiO2/MXene hetero-interfaces to avoid e−s from relocating back to TiO2. In addition to SB and fast e− shuttling, build-up of e−s on MXene will respond to H+-ions to create H2.

Adapted with permission from Ref. [242]
a–c Photo-catalytic H2 formation rates of TiO2/Ti3C2Tx and controlled samples. (a) Photo-catalytic HER activity of TiO2 loaded with particular metal carbide MXene co-catalysts (Ti3C2Tx, Ti2CTx, and Nb2CTx), (b) formation of SB at MXene/TiO2 hetero-interface, and (c) Wang et al. [240]. d Photo-catalytic HER of Nb2O5/C/Nb2C samples (Tongming Su et al. [242]). e–g Fabrication method of CdS/Ti3C2 cauliflower-structured sub-microsphere (e), charge separation and transfer in CdS/Ti3C2 system after vis-light illumination (red and blue spheres illustrate photo-induced e−s and h+s, correspondingly) (f) and at equilibrium potential HER free energy diagram (g).
In addition, Su et al. [242] formed Nb2O5/C/Nb2C (MXene) hybrid nano-composites (NCN-x) through oxidizing Nb2CTx surface at various durations (x = 0.5, 1, and 1.5 h) to produce a Nb2O5 layer via utilizing CO2 as gentle oxidant. Optimum duration of oxidation of 1 h photo-activity of NCN-1.0 for HER was four times higher as compared to pure Nb2O5 (Fig. 19d), which was created from close interfacial junction in conducting Nb2C co-catalysts and SC Nb2O5 for exceptional (e−–h+)-separation. This showed generation of transition metal carbides as feasible co-catalysts in solar to chemical energy conversion. Other than MO photo-catalysts, hybridization of TMDCs with MXene has also turned into an effervescent area of catalysis research in energy conversion. Qiao and co-workers also studied metal sulfide/Ti3C2 (metal sulfide: CdS, ZnS, and ZnxCd1−xS) nano-hybrid photo-catalysts (Fig. 19e) [243]. The CdS/Ti3C2 composite records high vis-light HER photo-activity (14,342 mol h−1 g−1, 136.6 times that of bare CdS) with surprisingly large apparent QY value 40.1% at 420 nm, showing that it was one of the most excellent noble metal-free metal sulfide photo-catalytic system. The DFT simulations showed that O2-terminated Ti3C2 was talented co-catalyst derived from its marvelous HER activity, outstanding metallic conductance, and enviable Fermi levels. Basically, such investigation formed a novel view for scheming high-efficient and cost-efficient solar H2O splitting utilizing metal chalcogenide photo-catalysts and photo-electrodes. Moreover, MXene-based composite with metal-free g-C3N4 photo-catalysts has a new start of attention in kingdom of renewable energy formation. Shao et al. [244] formed a Ti2C/g-C3N4 photo-catalyst by use of thermal annealing of melamine with the 2D-Ti2C NSs for the outstanding HER activity. Optimum 0.4 wt% of Ti2C provides an elevated H2-production rate of 950 mol h−1 g−1 with apparent QY of 4.3% at 420 nm. Enhanced photo-catalytic efficiency was observed by efficient charge transfer and separation due to the existence of SB to decrease H+ to H2. Similarly, Sun et al. [75] also studied g-C3N4/Ti3C2Tx photo-catalysts, in which Ti3C2Tx with O-surface terminations enhances the separation of charges for improvement of 105% in the HER activity with apparent QY of 1.27%. The DFT simulations support it and showed that O-terminated Ti3C2 with 25% H-atoms presents obvious free energy as low as 0.011 eV [241]. Giorgio Carraro et al. [81] explored enhanced H2 evolution via photo-reforming of sustainable oxygenates using nano-structure Fe2O3 polymorphs. They studied that Fe(III) oxide polymorphs, β- and ε-Fe2O3, have notable performance in solar spectrum for H2 production from solutions of renewable oxygenates (i.e., ethanol, glycerol, glucose). For β-Fe2O3 and ε-Fe2O3, H2 evolution rates up to 225 and 125 mmol h−1 m−2 are gained, along significant better activities in regard to commonly study α-Fe2O3.
6.3 Reduction of CO2
Other than photo-catalytic H2-evolution, CO2 reductions to produce hydrocarbon fuels over photo-catalysts were observed as an efficient way to concurrently reduce energy crisis and greenhouse cause. Basically, combustion of fossil fuel develops a great quantity of CO2, causes an increase in greenhouse effect due to unstoppable increase in CO2. Significantly, conversion of CO2 to valuable fuels is an alarming challenge in the recent time. Certainly, CO2 composed a fundamental C1 building element for chemical industries, but its thermodynamic stability and very high kinetic blocked its broad industrial uses. The undeniable solution for this mystery is to discover C-neutral fuels for low-C market to a sustainable future without environment disadvantages [245]. Usually, the CO2 molecules are very stable with C=O bond dissociation energy higher than ~ 750 kJ mol−1. Thus, in CO2 photo-reduction method, higher energy is required to split O=C=O structure, that is very demanding compared to H2O splitting into H2. Gateway for creation of a CO2·− intermediate through single e− transmission to activate a CO2 was observed as a rate-limited step to ensue proton-concerned reduction method. A theoretical potential of − 1.9 V versus NHE is needed for initial startup, and a superior over-potential is necessary for actual utilized potentials. Furthermore, multiple proton-coupled e− transfer methods are concerned in CO2 activation and reaction ways were quite difficult and varied with the synthesis of different products. In accordance with diverse number of injected e−s (2e−, 4e−, 6e−, and 8e−), the products, for example, are CO, HCHO, CH3OH, and CH4, correspondingly. Moreover, competition exists for CO2 photo-reduction and H2O reduction, because H2O reduction to produce H2 is energetically more encouraging that confines products selectivity. Current research explained that ultra-thin 2DMs-based photo-catalysts also displayed outstanding CO2 photo-reduction. For instance, Bi2WO6 layers with single-unit-cell thickness were formed through a lamellar hybrid intermediate plan [246]. Sodium oleate was used to provide oleate ions, thus to relate to Bi3+ via electrostatic interaction. Afterward, lamellar Bi-oleate complexes were formed through self-assembly with tail-to-tail or head-to-head bilayer array of oleate ions to construct a meso-structure. While Na2WO4 was inserted and treated with hydrothermal method, Bi2WO6 was formed and lamellar meso-structure was self-exfoliated into a single-unit-cell layer. As-synthesized single-unit-cell Bi2WO6 layer was used in photo-catalyst for CO2 photo-reduction and a 300 W Xe lamp. The Bi2WO6 powder was suspended in H2O along highly pure CO2 gas constantly bubbled during solution for measurements. An average rate of 75 µmol g−1 h−1 was obtained in methanol synthesis over single-unit-cell Bi2WO6 layers for 5-h analysis that was almost 3 and 125 times higher comparative to Bi2WO6 nano-crystals and bulk Bi2WO6 correspondingly. Following six cycles, photo-reduction effectiveness remains the same and is not effected by any deterioration and shows an outstanding photo-stability. Recent research initiates that an excellent photo-catalytic performance was resulting from the novel geometrical configuration of single-unit-cell Bi2WO6 layers, as follows:
-
1.
Initially ultra-large SSAs of single-unit-cell Bi2WO6 layers make sure 3 factors higher capacitance in CO2 adsorption that was a significant tip for CO2 activation and reduction.
-
2.
The single-unit-cell thicknesses give advance charge separation and extend carrier duration as testified through time-resolved fluorescence emission spectrum calculations.
-
3.
Single-unit-cell thickness carries almost higher DOS at CB edge as well as boosted surface charge density and hence promoted the 2D-conductivity.
Research certainly showed benefits of 2DMs photo-catalyst for CO2 photo-reduction. Adsorption of CO2 is a significant condition for CO2 photo-reduction, which considerably influences e− transfer method. Here, it is attractive to find suitable approach to boost adsorption site for CO2 adsorption and generate strong contact to make possible e−s transfer for efficient activation. Research concluded that generating surface defect sites is possibly another way [97]. Although with controlled synthesis, ZnAl-LDH ultra-thin NSs with thicknesses of 2.7 and 4.1 nm and bulk ZnAl-LDH with almost 210 nm thicknesses can be synthesized that are known as ZnAl−1, ZnAl−2, and ZnAl−3, correspondingly. While bulk ZnAl-LDH thickness is reduced to ultra-thin configuration, density of Vo defects is increased, so reduces coordination number of nearby Zn-ion and initiates several coordinative unsaturated Zn-ions. So, Zn+–Vo-complexes are built in ZnAl-LDH NSs as supported through XAFS, ESR, and PAS spectra calculations. In CO2 photo-reduction route, synthesized Zn+–Vo complexes can function as entrapping positions to encourage CO2 adsorption. Simultaneously, EIS and DFT simulation showed that Zn+–Vo complexes can provide e−s entrapping sites to improve charge separation effectiveness and make easy e−-transfer to CO2. Therefore, defect-rich ZnAl-LDH NSs showed great photo-catalytic activity for conversion of CO2 into CO through a 2e− method, with 7.6 µmol g−1 h−1 conversion efficiency for ZnAl-1. Further improved CO2 conversion competency, selectivity, and developed stability obtained by Xie et al. [154] tuned Vv into a single-unit-cell o-BiVO4 and then it is used as a photo-catalyst for CO2-reduction. Using cetyltrimethylammonium bromide, a lamellar hybrid half way plan was used for the formation of o-BiVO4 layers with single-unit-cell thickness. Regulating reaction time and temperature, Vv-rich and Vv-poor o-BiVO4 atomic layers were formed with [001] direction. Atomically thick o-BiVO4 NSs showed AFM and TEM results. As proved through PAS and XRF, Vv with discrete levels was formed on Vv-poor o-BiVO4 and Vv-rich o-BiVO4 NSs surface. Vv showed a significant function in photo-catalytic method for CO2-reduction. Firstly, Vv created a new defect concentration in BG and enhanced h+s level near the Fermi level. Therefore, light harvesting of Vv-rich o-BiVO4 was improved and electronic conductance was better. The Vv-rich o-BiVO4 showed an increased CO2-adsorption capacitance and stronger surface hydrophilicity comparative to Vv-poor o-BiVO4; this was certainly useful to CO2 reduction process. Finally, Vv improved charge separation and efficiently boosted carrier lifetime that permits more e−s to engage in CO2 photo-reduction. To get benefit from already discussed benefits, Vv-rich o-BiVO4 showed improved conversion effectiveness with methanol production rate of 398.3 µmol g−1 h−1. In photo-reduction method, just small concentration of H2 and trace amount of ethanol can be detected, signifying highly suitable method for product selection. Furthermore, the Vv-rich o-BiVO4 can undergo continuous photo-reduction reaction up to 96 h, lacking any clear decrease in the photo-catalytic efficiency. The above such outcomes show that the 2D photo-catalysts are efficient choice for getting highly efficient CO2 reduction. In case of pristine MXene, somehow the consideration of surface terminations (–OH and –O functional group) was very important to clarify reaction steps for CO2RR. In case of the Cr3C2 and Mo3C2 MXene, with no surface termination groups, energy input of 1.05 and 1.31 eV was essential for transfer of CO2 to CH4 (Fig. 20a). While Mo3C2 surface was terminated with OH or O, energy was further decreased to 0.35 and 0.54 eV, correspondingly. Therefore, OH or O-terminated MXene was certainly made easy CO2 conversion in comparison with un-functionalized MXene. Moreover, photo-catalytic reduction of CO2 at Vo on Ti2CO2, Ti3C2O2, and V2CO2 was studied theoretically by the first-principles DFT simulations [247]. These results showed that Ti2CO2 needs the minimum energy of reaction method and therefore proves good CO2RR activity. The CO2 was decreased to subsequent four compounds: HCHO, CH3OH, CH4, and HCOOH. Obviously, whole energy barrier for CO2 hydrogenation into HCOOH at Ov in Ti2CO2 SL was very good with just 0.53 eV compared with other reduction products that needed superior kinetics. For example, Ov on O-terminated MXene was active site in CO2RR for high HCOOH selectivity; therefore, MXene NSs can be used for broad range of applications.

Adapted with permission from Ref. [251]
a Lowest amount energy routes (PBE/DFT-D3 calculations) pursued for CO2 into *CH4 and **H2O conversion, catalyzed by Mo3C2. Gray, lilac, red, and white spheres indicate C, Mo, O, and H atoms, correspondingly (Li et al. [248]). b Photo-catalytic CO and CH4 evolution rates over P25, 5Pt/P25, 5TC/P25, and 5TC-OH/P25 (Ye et al. [249]). C–f Photo-catalytic CO2RR of the TiO2/Ti3C2 (TT-x) samples and P25 for the CH4 formation (c) and FE-SEM images of TT550 (d, e) and TT650 (f) (Low et al. [250]). g, h Photo-induced e− migration method at Ti3C2/Bi2WO6 hetero-interface (g) and photo-catalytic activity of Ti3C2/Bi2WO6 through diverse mass ratios of Ti3C2 to Bi2WO6 (0%, 0.5%, 1%, 2%, and 5%) (h).
Ye et al. [252] joined surface-alkalinized Ti3C2 MXene as co-catalysts with marketable P25 through a simple mechanical mixing technique for important improvement in photo-catalytic CO2RR. Following surface alkalinization, 5 wt% Ti3C2(OH)2-doped P25 (5TC-OH/P25) reveals obvious support in CH4 release as compared to un-modified 5TC/P25 (Fig. 20b). The DFT study showed that adsorption energy of CO2 on TC-F (F-termination) was superior to CO2 on TC-OH (OH-termination). Thus, CO2 molecules were easily adsorbed at TC-OH surface, leading to the synthesis of activated CO32−. So, encouraging charge separation, extraordinary electrical conductance, sufficient CO2 adsorption, and activation sites on alkalinized MXene were main things contributed to photo-catalytic improvement. These clearly showed the major job of surface alkalinization of MXene, as a valuable metal-free co-catalyst for synthetic photo-synthesis.
Similarly, Low et al. [253] studied in situ formed TiO2 NPs on conductive Ti3C2, to form TiO2/Ti3C2 hybrids (TT-x, where x shows the calcination temperature) through thermal annealing for CH4 production from CO2RR (Fig. 20c). After –F functional groups removal at elevated temperature, it brings oxidation of Ti3C2 tuned with –O functional groups. The TT550 and TT650 morphology was clearly different from Ti3C2 but analogous to rice crust (Fig. 20d–f). High conductivity of Ti3C2 promotes e−-transfer from TiO2 and exclusive rice crust analogue morphology with a large active site density, considerably push photo-catalytic activity. Referring to precede work which is discussed already [252], it was hard to straight evaluate both TiO2/Ti3C2Tx photo-catalytic systems due to different synthesis methods, unlike TiO2 phase, discrete difference in morphology, and diverse MXene surface modification. Therefore, it is sensible to embrace AQY for CO2RR as a controlled tool to describe different future experiment conditions. In recent times, same group of researchers has formed ultra-thin 2D/2D Ti3C2/Bi2WO6 hetero-junction hybrid nano-composites (Fig. 20g) [254]. Due to electronic coupling and intense physical effects, 2D/2D hetero-junction noticeably improves transfer and partition of photo-induced charge carriers to reduce charge recombination. 2 wt% Ti3C2-modified Bi2WO6 NSs (NSs) (TB2) records the highest CH4 release rate than other stoichiometry (Fig. 20h). Moreover, large interfacial contact surfaces of intimate 2D/2D hetero-junction donate more quick charge mobility in comparison with 0D/2D and 1D/2D hetero-junctions due to a decrease in charge transfer path. So, such stimulating investigation on parallel 2D/2D hetero-interfaces generates novel potential in material science for layered hetero-junctions design in electrocatalysis and photo-catalysis for energy conversion.
6.4 Nitrogen (N2) Fixation
In comparison with CO2RR, the photo-catalytic N2-fixation is still more demanding as dissociation enthalpy of N2 molecule triple bond (962 kJ mol−1). Normally, catalyst conversion for N2 is enormously harsh as N2 just weakly binds with solid-state catalysts and reaction entails high-energy intermediates. So, it is very much required to do an appropriate structure to support conversion competency of N2 to NH3. E0 to make the N2− is as high as − 4.2 V versus NHE via \({\text{N}}_{2} + {\text{e}}^{ - } \to {\text{N}}^{2 - }\) method, whereas proton-coupled e− transfer reaction \({\text{N}}_{2} + {\text{H}}^{ + } + {\text{e}}^{ - } \to {\text{N}}_{2} {\text{H}}\) has more available E0 of − 3.2 V versus NHE [255]. Proton-supported exchange method might evade production of high-energy intermediates and therefore reduce thermodynamic kinetics for NH3 formation. Current investigations showed that 2DMs are a talented applicant to get an efficient photo-catalytic N2 fixation [249]. Zhang and co-workers [250] studied that Vo formation in BiOBr NSs can efficiently boost N2 fixation reaction. Theoretical study showed that N2 adsorption onto the Vo through an end on arrangement of adsorbed N2 triple bond can be extended from 1.078 Å in original N2 to 1.133 Å, signifying efficient N2 activation. Generated e−s in BiOBr can simply inject into N2 π antibonding orbitals. Accessibility of localized π-back donating e−s in Vo might efficiently adsorb N2 to generate activation. So, N2 reduction to NH3 catalyzed through the BiOBr with Vo needs a very low reaction kinetic and a higher photo-catalytic performance can be obtained. N2 conversion rate after vis-light and UV–Vis-light irradiation is 104.2 and 223.3 µmol g−1 h−1, respectively, after without h+ scavenger or co-catalyst. Stimulated through Vo-motivated N2 activation, MoS2 NSs with S-vacancy were formed and further utilized to N2 fixation. The MoS2 NSs showed NH3 formation rate almost 325 µmol g−1 with 10-h measurement after simulated solar light irradiation. Although marketable bulk MoS2 cannot give any photo-catalytic performance for NH3 synthesis under similar test condition, it illustrates exclusive benefit of MoS2 NSs for N2-reduction. According to Mott–Schottky spectra, MoS2 NSs and bulk MoS2 samples in CB positions were anticipated to be − 0.35 and − 0.24 V, correspondingly, that were positioned below thermodynamic reduction potentials of N2 through one or two-e− transfer method. So, it was presumed that N2 reduction with MoS2 NSs was multi-electron coupled proton transfer method. Due to n-type SC essence, there subsists many free e−s in MoS2 NSs and such free e−s can pair with photo-generated excitons to form charge excitons (trions) that were mostly located around Mo-sites. Produced trions have manifold e−s in one bound state that was useful to multi-e− migration reactions. While N2 is confined through S-vacancies, it was bounded via three Mo atoms with trions after irradiation. N2 activated after e−s donation from bonding orbitals and accepting e−s to its antibonding orbitals results in a trion-supported six-electron reduction method. The significant photo-catalytic N2 fixation resides in building adsorption site in N2 molecule, e−-rich systems for e− donation, engineering band configuration through enough E0 and coupling protons to decrease energy condition of intermediates. So, theoretical and experimental study showed that novel breakthrough on MXene-based composites for CO2RR and N2RR will appear in the frontline of technology and science, transferring them in future real energy applications.
6.5 Organic Synthesis
Idea of decreasing energy expenditure for chemically developed, solar light-driven chemical transformation through assist of the SCs holds huge view. Under irradiation, SCs can use solar light to make an exciton or hot carriers that can stimulate chemical reactions at surface of catalyst. Effectiveness and selectivity of the photo-catalytic conversion are still in adequate at large scale. Weak interaction of O2-molecule with photo-catalysts surfaces, particularly a defect-free surface, is a serious matter for poor effectiveness of photo-catalytic organic formations. Reaction involves O2, which needs efficient interfacial e−-transfer, either directly as donor or indirectly as e−-acceptor [256]. Additional significant subject is poor selectivity that might be obtained from photo-generated h+s. Generally, generated h+s has strong oxidizing capacity and is accountable for nonselective over-oxidation. In recent times, the 2D photo-catalysts displayed a huge promise for selective organic transformation using mild conditions. For example, Xiong et al. [257] formed Vo into ultra-thin WO3 NSs to make O2-molecules active and activate organic conversion. Defect-rich (DR) WO3 NSs were formed through calcination of the first synthesized WO3·H2O NSs in N2-environment, at 673 K, whereas defect-deficient (DD) WO3 NSs were synthesized via calcination in atmospheric condition. From aberration-corrected HAADF-STEM, it is clear that DD WO3 shows comparatively smooth and flat surface. Simultaneously, continuous and controlled lattice fringe was experienced from atomic resolution HAADF-STEM image (Fig. 21). Such outcomes conclusively proposed the defects deficiency in such DD WO3 sample. In DR WO3, many small pits were formed, with small lattice disorder, and dislocation appears in NSs that vigorously emphasize the presence of different defects.

Adapted with permission from Ref. [257]
a–f Morphological study. g Fourier transforms W L3-edge EXAFS spectra with respect to commercial WO3. h ESR spectra at room temperature. i Scheme showing locations of Vo in WO3 lattice. j Cyclic analysis for defect-rich WO3 in catalytic aerobic coupling of benzylamine after irradiation with λ > 400 nm at 298 K. k Scheme shows total light-driven catalytic reaction route.
To further reveal the presence of Vo, synchrotron radiation-related XAFS spectroscopy was performed. The DR WO3 showed a clear diverse confined atomic configuration compared to DD WO3, where W-atom coordination numbers decrease from 6 to 5.4 for DD WO3 and DR WO3, showing local deficiency of O2-atom. ESR investigation was also used to calculate WO3 samples. Noticeably, at g = 2.002 a symmetric ESR signal was experimented for the DR WO3, showing e−-entrapping at Vo. Joint STEM, XAFS, and ESR findings suggested that subsistence of Vo at precise sites induces few lattice distortion and displacement. After getting benefits from DR configuration, O2 was chemisorbed at Vo of defect-rich WO3 NSs through end on arrangement and associated with e-s transfer from coordinative unsaturated site to O2. So, O2 was efficiently activated to O2·− species over DR WO3, and it was transformed from amines to respective imines with 6 times enhanced kinetic rate compared to DD WO3. As reaction time increased to 8 h as conversion ratio of benzylamine was greater compared to 80% with an extremely high selectivity, no clear decline was observed within 6 times of catalytic cycles. Other than O2-activation, hydrophobicity was another significant feature to affect photo-catalytic organic conversion method. Li and co-workers [258] studied colloidal formation approach information of BiOCl NSs. Through BiCl3 hydrolysis in octa-decylene solution, supported by in situ preparation of H2O via reaction in oleylamine and oleate solution, single-crystalline BiOCl colloidal NSs (BiOCl C-UTNSs) were obtained with almost 3.7 nm thickness. For comparison, BiOCl NSs were also formed through hydrothermal way, known as BiOCl H-UT-NSs. Surface H2O contact angle (CA) measurement was utilized to establish wettability of as-synthesized BiOCl NSs. BiOCl C-UT-NSs showed a H2O CA of 116.3°, which is hydrophobic. It occurs from detail that organic ligands have capped on BiOCl C-UT-NSs surface during colloidal formation. Conversely, BiOCl H-UT-NSs exhibited a H2O CA of 0°, showing the super-hydrophilic nature of synthesized BiOCl H-UT-NSs. Huge difference in BiOCl C-UT-NSs and BiOCl H-UT-NSs may bring about important effect for photo-catalytic organic conversion method. Moreover, there subsist plentiful Vo on BiOCl C-UT-NSs, ensuing strong light absorption in vis-light range. To get benefit from hydrophobic character and enhanced light harvesting capability, BiOCl C-UT-NSs displayed greatly enhanced photo-catalytic activity for conversion of N-t-butylbenzylamine to N-t-butyl-benzylamine. The 78% conversion ratio was obtained from BiOCl C-UT-NSs, whereas BiOCl H-UT-NSs have only displayed about 15% conversion rate, from Xe lamp irradiation for 1 h. Furthermore, BiOCl C-UT-NS sample was more utilized for conversion of secondary amines to respective imines along increased conversion selectivity as well as efficiency. The 2D photo-catalysts were verified to be talented choice for the photo-catalytic organic formation, and such approach can broaden perceptive of organic conversion method and is favorable to establish further proficient organic transformation systems.
6.6 Removal of Pollutants
In the progress of financial and industrialization, environmental pollution is the main problem that threatens the public health. The photo-catalysis was considered as an efficient and financially viable technology to handle elimination of environmental pollutions. Due to exclusive advantages such as better adsorption capability of pollutants and strong light harvesting capacitance, 2DMs-based photo-catalysts showed a great hope for removal of pollutants. For instance, Xia and co-workers [140] formed ultra-thin Bi4O5Br2 NSs through reactive ionic liquid supported via solvothermal method in combination with pH adjustment. In ionic liquids, long carbon chain served as a capping reagent which controls the crystal growth along c-axis. Simultaneously, reaction condition pH was adjusted to 11 that supplied OH− to substitute Br- to execute de-halogenation in fabrication route of Bi4O5Br2. So, both thickness and component-engineered Bi4O5Br2 materials were formed and utilized in photo-catalytic degradation of an antibiotic CIP and tetracycline (TC) after irradiation with vis-light for 120 min. After irradiation, 75% of CIP is photo-degraded through Bi4O5Br2, whereas BiOBr degradation rate was 51.4%. Furthermore, Bi4O5Br2 NSs showed 77.8% degradation rate for TC within 60-min irradiation, which is very high compared to BiOBr with just 31.7%. Changeable energy band configuration of Bi4O5Br2 was verified to consider the increased photo-catalytic activity. More negative CB position of Bi4O5Br2 will make easy development of more active O ·−2 species. Upshifting of CB position and broad VB will be advantageous to improve charge separation effectiveness. Thus, obtained Bi4O5Br2 NSs displayed greater performance toward pollutant removal. To further enhance photo-catalytic performance in pollutant degradation, creating surface defects might be another approach. Therefore, Xie and co-workers [119] studied that while BiOCl thickness is reduced from 30 to 2.7 nm, in BiOCl defect type will vary from isolate defects VBi″′ to triple vacancy-related VBi″′VO··VBi″′, as confirmed with PAS. Through desirable quality from triple vacancy-associated VBi″′VO··VBi″′ along four negative charges, BiOCl NSs were additionally negatively charged as comparative to BiOCl nano-plates. As RhB was positively charged, the further negatively charged ultra-thin BiOCl NSs promoted RhB adsorption on BiOCl NSs’ surface. Moreover, the presence of vacancies can enhance light absorption and speed up separation of charges. As a result, ultra-thin BiOCl NSs exhibited a great solar photo-catalytic activity for RhB removal. MXene shows outstanding performance in photo-catalytic degradation of organic pollutants. Mashtalir et al. [135] utilized Ti3C2Tx to degrade MB (a cationic dye) and acid blue 80 (AB80) (an anionic dye) (Fig. 22a, b). The MB and AB80 degradation was augmented via UV irradiation. In dark, MB level reduces due to negatively charged adsorption at Ti3C2Tx surfaces with MB. After UV irradiation, a substantial reduction in MB and AB80 concentration, with 81% and 62%, respectively, was experienced in existence of suspended Ti3C2Tx. It is observed that over longtime period, Ti3C2Tx oxidation to form TiO2 in dissolved O2 presence was obvious that merits wide ranging research in this field. Similar to G-TiO2 nano-composites, it was imagined that Ti3C2Tx-supported TiO2 may function as a possible catalyst that further promotes progress of this direction. While continuing such investigation for the design of hetero-junction interfaces, Peng et al. [259] studied a composite of Ti3C2 and {001} facets-exposed TiO2 through hydrothermal incomplete oxidation of Ti3C2 (Fig. 22c–e).

Adapted with permission from Ref. [259]
Photo-catalytic pollutant degradation utilizing MXenes and their hybrid nano-composites. a, b Time-dependent MB respective level (a) and AB80 (b) in Ti3C2Tx. Adapted with permission from Ref. [135]). c–e Preparation of (001)TiO2/Ti3C2 nano-hybrids (c), charge transfer process (d), B.G. (e) of (001)TiO2/Ti3C2 after light irradiation.
In photo-catalytic reaction, TiO2 through preferential {001} facets creates e−s and h+s through UV light illumination and, afterward, Ti3C2 develops a Schottky junction with {001}-face n-type TiO2. It hinders recombination of e−s with h+s due to SB. Enriched e−s on TiO2 (001) might interact with dissolved O2 to generate superoxide radical anions (O2−) that further respond to H+ and e− to produce extremely reactive hydroxyl (OH) radicals, which increase photo-degradation. It is well recognized that photo-catalytic activity of TiO2 relies not only on particle shape but also on its exposed facets. It seems that {001} surface offers oxidation sites in photo-catalytic method, while {101} facets proceed as reductive sites. Therefore, TiO2 facet tuning using MXene has basic significance to systematically untangle underlying photo-catalytic system [241]. In addition, TiO2 metal sulfides are also utilized to join with Ti3C2Tx. Xie et al. [260] formed a 2D in-plane CdS/Ti3C2Tx onto sheet hetero-structures via electrostatic self-assembly method (Fig. 23a). In such catalytic system, the Ti3C2Tx Janus co-catalysts not just act as an e− mediator to augment e−s extraction from CdS but also restrain h+-mediated photo-corrosion of CdS. Assigning Ti3C2Tx small Fermi level than CdS CB, photo-e− lifetime of CdS/0.5% Ti3C2Tx was longer as compared to bare CdS.
a Scheme of CdS/Ti3C2Tx NSs to improved photo-activity and photo-stability. b Adsorption of Cd2+ over CdS NSs and Ti3C2Tx MXene in dark. Adapted with permission from Ref. [261]. c Charge migration. d Separation and reaction mechanism for MO degradation in In2S3/anatase TiO2@metallic Ti3C2Tx (InTi) system after vis-light and photo-catalytic degradation of MO over other In2S3-based binary hybrids. e In2S3, In Ti hybrids, and Ti3C2Tx. f In Ti-x indicates mass of Ti3C2Tx included during preparation (x = 8, 16, 32, and 54 mg). Adapted with permission from Ref. [262]
Moreover, Ti3C2Tx can absorb Cd2+ ions that were produced during photo-catalysis (Fig. 23b) and, as a result, avoid Cd2+-ions dissolution in H2O to enhance photo-stability of CdS. For instance, double-gain strategy offers a conceptual idea to evade instability as well as photo-corrosion of CdS. Other than MXene-based binary hybrid nano-composites, prosperous study into ternary hetero-structures has become a mainstream in photo-catalysis area. Wang et al. [262] (2018) formed a new quasi-core–shell In2S3/anatase TiO2@metallic Ti3C2Tx MXene hetero-structure hybrids through in situ hydrothermal technique for degradation improvement in methyl orange (MO). Fascinatingly, enhanced photo-activity of ternary nano-architectures was credited to numerous associated factors, such as well-designed type II band arrangement and noble metal-free-based Schottky junction with promising charge migrating channels (Fig. 23c, d). Specially, such occurrence originates from synergistic contribution in between vis-light-responsive In2S3, upward band bending in TiO2, and amazing electrical Ti3C2Tx conductance. For example, optimal photo-catalyst in the presence of Ti3C2Tx content of 16 mg (InTi-16) evidents the highest photo-degradation competency for MO elimination, as well as more significantly, it exceeds other In2S3-based binary NSs (Fig. 23e, f). Progressing via this analysis, it can be elucidated that key to ameliorate dyes photo-catalytic degradation is to effectively separate photo-induced (e−–h+)-pairs to hold back charge recombination. Consequently, efficiently tuned vigorous hetero-structure system with distinct quality (e.g., intimate contacted hetero-interfaces, use broad solar spectrum to imitate natural sunlight, superior conductance, constructive charge transfer, and separation) is an urgent demand for boosting photo-catalytic degradation. Incidentally, extensive research in chemistry, materials arrangement, and optimization is the main requirement. In turn, it will open new prospects for separating charge carrier dynamics in synergistically speeded up photo-activity in practical photo-catalytic applications.
6.7 Hydrogen Peroxide (H2O2) Production
Since the first synthesis of H2O2 by Thenard (1818) as a result of barium peroxide reaction with nitric acid [263], H2O2 has gotten rising consideration in past 200 years due to which it was listed among the 100 most significant chemicals in world [264]. The anthraquinone oxidation (AQ) is mostly developed for H2O2 manufacture on industry level that is presently caused about 95% of total H2O2 formation. Normally, AQ method generally contains four steps [265]:
-
1.
Hydrogenation of AQ in organic solvent by means of Ni/Pd catalyst.
-
2.
Oxidation of hydrogenized AQ (HAQ) in air or O2-enriched atmosphere with the help of catalysts.
-
3.
Removal of H2O2 and recycling HAQ to AQ.
-
4.
Refinement and concentration of H2O2.
The multi-step oxidation and hydrogenation response need an elevated applied energy. On the other hand, AO method is not environmentally benevolent, since large quantity of waste H2O (for example, 2-ethyl-anthraquinone, tri-octyl phosphate, tert-butyl urea, and K2CO3 lye), exhaust gas (mesitylene isomers), and solid waste (activated alumina) was formed. It is well known that H2O2 is a very competent and ecological oxidant. It has maximum content of active O2 (47.1% w/w), and no noxious side products are formed in its reactions, apart from H2O and O2. Due to these qualities, H2O2 has broadly applied in organic synthesis, [266] waste H2O management, disinfection [267], and paper industry [268]. Nowadays, H2O2 is studied in energy field as both oxidant and reductant in innovative fuel cell [269]. The results illustrated that theoretical output potential of the H2O2 fuel cell was 1.09 V that is analogous to traditional H2-fuel cell (1.23 V). The H2O2 has established rising concentration as it is not only a mild and environment friendly oxidant but also a talented novel liquid fuel. Formation of H2O2 by photo-catalysis is green, sustainable, and potential method, in view of its utilization in H2O and O2 as source materials and solar light as energy. Other advantage of H2O2 as compared to H2 is that it is completely soluble in H2O and simply transportable that paves it as energy carrier perfectly.
Synthetic photo-synthesis is a photochemical method to renovate sustainable resources into clean fuels and chemicals (for example, H2O2) through sunlight which was expected to solve rising energy requirements. Photo-catalytic-based H2O2 formation is a talented approach to improve energy requirements, as H2O2 is significant liquid chemical and fuel. However, subsequent dilemma strictly limits the growth of this method:
-
1.
Less selectivity.
-
2.
Less stability (usually > 5 short-time cycles).
-
3.
Quick charge recombination.
-
4.
Support of hole scavengers.
-
5.
Requirement of O2 saturation.
TiO2 is broadly considered as a photo-catalyst because of its crystal stability, optical, physical and electrical properties as well as biocompatibility [17, 18]. H2O2 manufactured by TiO2 photo-catalytic attracted a great concentration [19]. The TiO2 CB (− 0.19 V vs. NHE, pH 0) bottom is more negative than 2e− reduction of O2 (0.68 V), which promotes reduction reactions for H2O2 fabrication, but pristine TiO2 has few disadvantages for example, poor light absorption due to its large BG. Mainly, quantity of H2O2 generation is much low (< 0.2 mM) over pristine TiO2-based photo-catalyst that is away from reasonable level. This may be after the formation of H2O2; it straight away reacts with surface Ti–OH groups and forms Ti–OOH complexes. Secondly, Ti-OOH complexes dissociated to Ti–OH and OH− through e− reduction as:
Numerous surface alteration approaches were applied to boost photo-catalytic fabrication of H2O2 in TiO2-based photo-catalytic method, for example surface fluorination and surface complexation. The g-C3N4 is analog to graphite, and metal-free polymer n-type SCs have stacked 2D configuration of tri-s-triazine linked through tertiary amines [38]. In consequence of its exceptional structural, electrical, optical, and physicochemical properties, g-C3N4 is familiar as a novel class of multipurpose materials for catalytic, electronic, and energy uses [39]. Wang and co-authors (2009) first revealed photo-catalytic properties of g-C3N4 on H2 and O2 evolution [40], and g-C3N4-based photo-catalysts have attracted boosting interest worldwide [41]. BG of g-C3N4 is ~ 2.7 eV which is similar to optical wavelength of ~ 460 nm, that makes it a possible vis-light-active photo-catalyst. Additionally, g-C3N4 has photo-catalytic capability for H2O reduction and oxidation because of its suitable BGs [42]. Hypothetically, g-C3N4 is a good photo-catalyst applicant for H2O2 formation [10] as its CB position (− 1.3 V vs. NHE) is correctly located to make possible O2 reduction (− 0.28 V vs. NHE), while VB potential (1.4 V vs. NHE) is smaller compared to metal oxides that can efficiently avoid oxidative disintegration of H2O2. As such, g-C3N4 rapidly becomes attractive in the field of photo-catalysis H2O2 formation [43]. However, photo-catalytic H2O2 formation activity of g-C3N4 is still limited via low effectiveness in consequence of some adverse parameters, generally including lower surface area, inadequate vis-light harvesting, and quick recombination of photo-induced (e−–h+)-pairs. Protocols, for instance engineering structures, controlled defects, loaded noble metal nanoparticles, doping elementals, and heterogenization, were later applied to improve g-C3N4-based photo-catalytic H2O2 formation.
7 2D/2D Hetero-junctions for Catalysis
The description of hetero-junction, initially developed from SC–SC (S–S) junction, now has been elaborated to scope metal–SC (M-S) junction and still nontypical hetero-structures of SCs and ionic conductors [136]. Mostly, edge coupling of two components in a hetero-junction could make band arrangement or repairing contact after Fermi levels equilibration (or work functions) at interface following Anderson’s rule or Schottky–Mott rule for S–S or M–S junctions, correspondingly. There is an agreement in previous work that re-localization of charge carriers at hetero-junctions edge may make easy catalytic activity of as-fabricated materials or devices.
7.1 Photo-catalytic H2 Production
Since 1972, Fujishima and Honda [37] discovered H2O splitting at TiO2 electrode under UV light irradiation; at the same time, numerous efforts were dedicated to photo-catalytic H2-production [136]. The photo-catalytic is a procedure that produces H2 (and O2) using reduced or oxidized adsorbed H2O through photo-generated e−s and h+s at SC catalysts’ surface. Due to quick recombination of photo-generated (e−–h+) in catalysts, for H2 production effectiveness was still far from prerequisite practical applications. Therefore, 2D/2D layered composite photo-catalysts with suitably formed hetero-junctions are moderately promising for increasing H2-production effectiveness via supporting separation of photo-generated (e−–h+) [270]. In comparison with UV light with small percentage of the solar emission, vis-light (almost 50% of solar radiation) determined that the photo-catalysts are more capable for high proficient sunlight utilization and photo-catalytic activity. Zhang et al. [271] developed a type of “sheet on sheet” hierarchical hetero-structure for vis-light-based photo-catalytic H2-production via in situ development of ZnIn2S4 2D-NSs on sheetlike g-C3N4 surfaces. g-C3N4 was one of the most talented photo-catalysts because of its good stability, non-toxicity, exceptional electronic configuration, and cost efficiency. Its photo-catalytic effectiveness is restricted through poor light harvesting effectiveness and quick recombination of photo-generated charge carriers. Combining vis-active ZnIn2S4 2D-NSs, the above shortcomings were overcome based on hetero-junction contact interface that can persuade proficient interfacial transfer of photo-generated (e−–h+) from g-C3N4 to ZnIn2S4 and delayed charge recombination depending on measurement findings of surface photo-voltage and PL of ZnIn2S4/g-C3N4 hetero-structures. Both suppressed charge recombination on g-C3N4 NSs and enhanced photo-generated charge carriers in ZnIn2S4 NSs give amazing improvement on photo-catalytic activity of ZnIn2S4/g-C3N4 hetero-structures for H2-production.
Enhanced photo-catalytic activity should also be contributed to increase in surface active sites and extension of light absorption via ZnIn2S4 NSs combination. The overall photo-catalysts-based H2O splitting was also developed for production of O2 and H2 concurrently, but it is still a major confront [270]. To fulfill redox potential for overall H2O splitting, photo-catalysts CBM must more negative as compared to E0 of H+/H2 (0 V vs. normal H2 electrode (NHE)) and VBM should be more positive compared to oxidation potential of O2/H2O (1.23 V). Over-potential related to charge transfer and minimum BG of photo-catalysts for efficient H2O splitting is always superior compared to theoretical value (1.23 eV). Therefore, Liao et al. formed 2D MoS2/AlN(GaN)-layered hetero-structures as extremely competent vis-light photo-catalysts for overall H2O splitting. The H2 and O2 were formed at opposite surfaces of hetero-structures, because AlN (GaN) and MoS2 SLs act as e− donor and e− acceptor in this hetero-junction photo-catalyst, respectively. Pristine MoS2 using a direct BG of 1.9 eV was a potential vis-light-driven photo-catalyst and is confirmed not efficient in H2O splitting. Group III nitrides (AlN or GaN) SLs with good thermal/chemical stability and highly thermal conductance have demonstrated a good option to manufacture SLs MoS2 hetero-structures, to improve photo-catalytic activity. Moreover, there was only around 2% lattice mismatch among h-AlN (GaN) and MoS2 SLs, which was major benefit for manufacturing of hetero-structures. The MoS2/AlN and MoS2/GaN hetero-structures were calculated to be capable photo-catalysts under vis-light irradiation because of proper BGs for H2O splitting and good optical absorption [136].
In recent times, polymers were supposed to be one of the potential alternatives in photo-catalytic overall H2O splitting because of its engineering molecular structures [272]. To remove limitation of single-element polymer with inadequate E0, 2D/2D polymer hetero-junction photo-catalysts with Z-scheme configuration were designed from imitating two-step excitation way of plants, where light-driven two half reactions of H2O splitting and glucose formation are divided spatially [273]. The Z-scheme-based polymer derived from 2DMs was proved to be an effective pathway to get appropriate energy levels for enough reaction kinetics and allow proficient charge transfer in overall H2O splitting. Wang et al. [270] synthesized hetero-structures (aza-CMP/C2N) consisting of aza-conjugated microporous polymers (CMP) and C2N NSs as 2D polymer-related Z-scheme systems for competent photo-catalytic overall H2O splitting. The aza-CMP/C2N hetero-structures were synthesized via mixing and consequently annealing of CMP and C2N NSs. The as-fabricated stacked NSs of aza-CMP/C2N with abundant overlapped areas might be exposed via the TEM analysis in Fig. 24a. The XANES spectroscopy, HAADF-STEM image, and elemental mapping were utilized to show interlayer relations as well as homogeneous combination of aza-CMP and C2N NSs (Fig. 24b).

Adapted with permission from Ref. [274]
a TEM image. b NK-edge XANES of aza-CMP, C2N, and aza-CMP/C2N. c HAADF-STEM images and elemental mapping (Pt-labeled C2N and Co-labeled aza-CMP). d Overall H2O splitting activity. e Scheme showing aza-CMP/C2N hetero-structures electronic band structures. f J–V curves of catalysts in dark and under light irradiation.
Investigation on photo-catalytic activity showed that H2 and O2 (molar ratio 2:1) were concurrently formed from pristine H2O with aza-CMP/C2N hetero-structures as photo-catalysts under vis-light (> 420 nm) irradiation, whereas both aza-CMP and C2N components are inactive. In addition, aza-CMP/C2N hetero-structures with 1:1 mass ratio showed optimum photo-catalytic performance (H2 evolution rate: 5.0 mmol h−1, solar to H2 conversion efficiency: 0.23%, evident QE at 600 nm: 4.3%) (Fig. 24c). Figure 24d presents the energy band position of aza-CMP/C2N hetero-structures, which indicates that photo-generated e−s in C > B of aza-CMP were quickly recombined with photo-generated h+s at VB, of C2N at their interface, while other photo-generated e−s and h+s participated in H2O redox reaction. Credit to as-constructed hetero-structures, both charge separation and transfer were assisted in aza-CMP/C2N composites (confirmed via outcomes of electrochemical impedance measurements and transient photo-current) and therefore increased photo-catalytic performance.
As a move toward green chemistry, sequence of metal-free photo-catalysts, e.g., g-C3N4, BP, boron nitride (BN), and boron carbide (BC), was highly preferred to be studied because of their profusion, inexpensive, and excellent stability. The study of a competent and stable metal-free photo-catalyst with broad spectrum solar absorption for photo-catalytic H2-production remains a major issue. Zhu et al. [275] productively formed a 2D layered hetero-structures consisting of BP and graphitic carbon nitride (CN) (BP/CN) as metal-free photo-catalysts in H2-evolution for vis to near-IR region for the very first time. Compared to a single element (BP or CN), BP/CN hetero-structures showed considerably enhanced photo-catalytic activity, and H2-evolution for 3 h obtained 1.93 µmol, 0.46 µmol under > 420 nm > 780 nm light irradiation, respectively (Fig. 25a, d). It is mostly due to proficient interface charge transfer based on strong interface interaction in CN and BP that restrained recombination and improved the partition of photo-generated (e−–h+)-pairs (Fig. 25f). After excitation in vis-light, a charge migration from CN to combine BP stimulated through hetero-junction interfacial effect in BP/CN can be established through consequences of time-resolved diffuse reflectance spectroscopy. In NIR excitation case, just BP was excited and extensive excitation duration is obtained from competent electron entrapping via P-N coordinate bond at hetero-junction edge [136].

Adapted with permission from Ref. [275]
a Photo-catalytic H2 evolution based on different catalysts with > 420 nm. b Effect of BP/CN ratio in BP/CN on photo-catalytic H2 production rate under vis-light irradiation for 3 h. c Cycle stability test on BP/CN photo-catalytic H2 production under vis-light irradiation. d Photo-catalytic H2 production based on BP/CN with > 780 nm light irradiation. e HAADF-STEM image. f Scheme for photo-catalytic H2 evolution utilizing BP/CN.
Jingrun Ran et al. [276] studied metal-free photo-catalysts of metal-free 2D/2D vdWs phosphorene/g-C3N4 hetero-structure. The synthesized nano-composite showed improved vis-light photo-catalytic H2 production activity of ~ 571 µmol h−1 g−1 in 18 v % lactic acid aqueous solution. This enhanced activity occurs due to intimate electronic coupling at 2D/2D interface, which introduced a new metal-free phosphorene/g-C3N4 photo-catalyst and formed 2D/2D vdWs hetero-junction for uses in catalysis, electronics, and optoelectronics. Qixiao Gai et al. [277] synthesized 2D CdS and 2D CoP NSs, oxidation and phosphidation process. Then, 2D–2D CdSe/CoP photo-catalysts were formed by ultrasonically dispersing the mixed solution of CdS and CoP. The CdSe/CoP with 2% CoP loading amounts showed a maximum photo-catalytic performance of 56.3 mmol g−1 h−1 under vis-light irradiation that is 11.3 times as high as bare CdS. The improved photo-catalytic activity of CdS eCoP should be due to the following two points: (1) high catalytic activity of CoP; (2) highly proficient separation and transfer of (e−–h+)-pairs photo-generated in CdS because of synthesized 2D–2D hetero-structure. In Table 1, H2-production-based photo-catalysts are summarized.
7.2 Photo-catalytic Pollutant Degradation
In industrial progress, mass discharge of poisonous wastes (e.g., agrochemicals, dyes, and antibiotics) [136] has become worldwide a rigorous hazard to H2O resources and human physical condition [298]. Besides civilizing environment policies and system, development of an eco-friendly solution for eradicating pollution is dreadfully in demand. Among possible solutions, photo-catalytic decomposition of organic contaminants via in situ very reactive species is supposed to be green, cost-efficient, and talented move to deal with pollution matters. Even though numerous types of single ingredient photo-catalysts were developed, mostly suffer from a poor photo-catalytic activity and are not sufficiently efficient for real applications. A series of 2D/2D layer hetero-structures was generated for organic photo-degradation, showing potential uses. The 2D/2D hetero-structures consisting of AgIO3 and g-C3N4 NSs (AgIO3/g-C3N4-NS) were successfully formed for photo-catalytic waste H2O treatment after vis-light exposure by Li et al. [299]. The ultra-thin g-C3N4 NSs as polymeric organic SCs material were exhibiting superior vis-light response. Photo-catalytic performance of AgIO3/g-C3N4 NSs hetero-structures was considerably higher than that of single AgIO3 or g-C3N4 NSs for organic dyes degradation. Noticeably, degradation reaction rate constant of rhodamine B (RhB) than as-synthesized AgIO3/g-C3N4-NS sample was approximately 22.86 times higher as compared to hetero-structures composed of AgIO3 and bulk g-C3N4 (AgIO3/g-C3N4-B). It shows significance of 2DMs for construction of hetero-junction photo-catalysts (Fig. 26b).

Adapted with permission from Ref. [299]
a Scheme showing AgIO3/g-C3N4-NSs composite synthesis. b Photo-catalytic degradation kinetics of RhB after vis-light irradiation. c Scheme showing charge separation at AgIO3/g-C3N4-NS interface after vis-light irradiation.
A possible photo-catalytic method was also proposed in this work: The photo-induced e−s on CB of g-C3N4-NS can move to AgIO3 CB under vis-light irradiation that promoted photo-generated (e−–h+)-pairs separation in g-C3N4-NS (Fig. 26c). Considerably decreased steady-state PL spectra peak intensity and enhanced lifetime of charge carriers in PL spectra of AgIO3/g-C3N4 NSs as compared with that of single C3N4 NSs indicated superior e−-migration and charge separation effectiveness at hetero-junction edge. To consider O2/·O2− reduction potential, the oxidative breakdown of the dye should be due to h+s in VB of the g-C3N4 NSs and •O2− produced through e−s reduction of O2 from CB of g-C3N4 NSs. In another work, diverse quantity of BiOCl nano-plates is utilized to combine with C3N4 NSs via an easy calcination method. The face-to-face interaction edge of as-synthesized BiOCl/C3N4 hetero-structures was studied, and the significance of contact area of two components was discussed. The superior photo-current intensity and weakened PL intensity of BiOCl/C3N4 hetero-structures as compared with those of C3N4 NSs illustrated separation and migration of photo-generated e-s at interface. The BiOCl/C3N4 hetero-junction photo-catalysts were loaded with 70% BiOCl which showed the highest MO photo-degradation activity in vis-light irradiation. Wang et al. [300] prepared g-C3N4/Bi2WO6 2D/2D hetero-structures consisting of g-C3N4 NSs and SL Bi2WO6 NSs for degradation of ibuprofen under vis-light irradiation. It shows that highly active photo-degradation system might be developed via navigation of charge division, transportation, and consumption at atomic level. Bera et al. [301] prepared a chain of hetero-structures consisting of rGO and CdS with diverse dimensionality (rGO/CdS) for photo-catalytic degradation of methylene blue (MB).
The 2D-G as an example of 2D-layered material, whose atomic thickness has outstanding charge transfer ability, can offer conducting e−-channels for separation of the photo-generated charges in the hetero-junction photo-catalyst composed of BG and SC. Through utilizing terephthalic acid (TA) as an example, the OH• radicals were established to be generated active species for the photo-catalytic decomposition. Therefore, improved rGO/CdS activity in comparison with the single CdS might be explained as follows: Under vis-light irradiation, photo-generated e− in CB of CdS is transferred to rGO surface and reacts with adsorbed O2 that consequently produces ·O2− and OH·. Oxidization of both OH• and photo-generated h+s leads to photo-catalytic decomposition of MB dye molecules. Charge migration from CdS to rGO takes place at rGO/CdS hetero-structures interface, as proved from considerably quenched PL of CdS components, promoting separation of photo-generated charges and therefore enhancing photo-catalytic activity [136]. In a few cases, the photo-catalytic activity of pollutant degradation might be increased through synergetic effect in hetero-junctions and other configuration tuning.
Yangyang Liu et al. [302] studied a 2D/2D nano-composite photo-catalyst (ZnO/MoS2) derived from P-doped ZnO NSs with large SSA and 2D-MoS2 for competent photo-degradation of organic dyes (Fig. 27a–f). The ZnO/MoS2 hetero-structures with different MoS2 loading amounts (mass ratio is 0, 0.01, 0.1, and 1 wt%) and commercial P25 are utilized as photo-catalysts for photo-degradation calculations that took place in similar experimental conditions (Fig. 27d, e). Comparative findings illustrate that ZnO/MoS2 with a small loading amount of MoS2 (0.1 wt%) would considerably improve photo-catalytic activity in comparison with pristine ZnO NSs. When MoS2 loading amount was increased from 0.01 to 0.1 wt%, photo-catalytic performance of ZnO/MoS2 was further improved, but reduced after loading amount of MoS2 and further enhanced to 1 wt%. A huge MoS2-doped concentration would block sunlight that was utilized to force photo-degradation of MB and therefore decreases the photo-catalytic performance. A photo-catalytic mechanism of ZnO/MoS2 hetero-structures is proposed in Fig. 27f [136]. Derived from interfacial effect between ZnO and MoS2, photo-generated e+s would migrate from CB of ZnO to that of MoS2 that considerably improved the separation of carriers and therefore increase catalytic activity. Increased transport and separation of photo-generated h+s and e−s stimulated through interfacial effect can be established through increased photo-current density of MoS2/ZnO hetero-structures after introduction of MoS2 components. The photo-generated charges react with O2 and H2O after relocated to catalyst surface and generated much reactive radicals (OH and superoxide anion radicals) to degrade dye molecules. In addition to hetero-junction effect, P loading stimulated defects in ZnO NSs also support photo-catalytic activity as they can enhance light absorption via introduction of energy level between BGs [136].

Adapted with permission from Ref. [302]
a ln (C/C0) versus time curves of MB with different photo-catalysts: ZnO/MoS2 hetero-structures with diverse doping amounts of MoS2 (mass ratio is 0, 0.01, 0.1, and 1 wt%) and commercially available P25. b Obvious rate constants of MB photo-degradation with different photo-catalysts. c Scheme showing photo-catalytic system of ZnO/MoS2 hetero-structures.
7.3 Photo-catalytic CO2 Reduction
The 2D materials aptitudes to boost the specific surface area to give elevated surface reactive sites make them a top precedence for photo-catalyst supports. Provided large contact area of 2D/2D composite, contact in graphene and photo-catalyst is anticipated to be enhanced that speeds up transfer and separation of photo-generated (e−–h+)-pairs, therefore enhancing their photo-catalytic CO2 reduction activity. According to the above-discussed phenomena, J. Sun et al. [257] fabricated three classes of 2D/0D, 2D/1D, and 2D/2D graphene/TiO2 hetero-structures through solvothermal method. So, the 2D/2D graphene/TiO2 hetero-structure showed maximum photo-catalytic effectiveness in contrast to 0D/2D and 1D/2D graphene/TiO2 hetero-structures and pristine TiO2 NSs. This performance was ascribed due to stronger electronic and physical coupling in 2D/2D structure that provides more proficient electron transport (Fig. 28a–c). Also, Wee-Jun Ong et al. [303] studied 2D/2D sandwich-like graphene-g-C3N4 (GCN) composite formed by one-pot impregnation thermal reduction technique. It also shows a high photo-catalytic activity for CO2 reduction to manufacture CH4. The large contact interface in graphene and g-C3N4 plays a significant function to improve photo-catalytic CO2 reduction by rising electron movement (Fig. 28d–f).
a TiO2 and graphene NS structures. b TEM and element mapping of graphene/TiO2 hetero-structure. c Photo-catalytic degradation of rhodamine B (RhB) over different photo-catalysts under irradiation of UV light. Adapted with permission from Ref. [304]. d Preparation of GCN samples through one-pot impregnation–thermal reduction approach. e Total CH4 yield over as-synthesized photo-catalysts. f Photo-generated charge transfer in GCN system for CO2 reduction with H2O to make CH4. Adapted with permission from Ref. [303]
Furthermore, W. J. Ong et al. formed 2D/2D layered nano-structure of rGO/g-C3N4 by surface charge modification and protonation for improved photo-catalytic reduction of CO2 to CH4 [305]. The preparations involved ultrasonic dispersion with NaBH4-reduction method (Fig. 29a). In comparison with pristine g-C3N4 and rGO/CN, optimized composite 15 wt% rGO/pCN (15rGO/pCN) showed high CH4 production of 13.93 mmol g−1cat with a photochemical QY of 0.560% (Fig. 29b, c). Since g-C3N4 also showed 2D p-conjugated structure, this quality improves the capability of catalyst to adsorb CO2 molecules. When electrostatically charged with 2D conducting material rGO, pCN, and rGO created efficient interface, which caused improved performance for CO2 reduction (Fig. 29d).

Adapted with permission from Ref. [305]
a Production method of rGO/pCN. b Total production of CH4 over pure g-C3N4 and a series of rGO/pCN photo-catalysts with different rGO contents under vis-light irradiation for 10 h. c Total evolution of CH4 after 10 h over rGO, pure g-C3N4, pCN, and 15rGO/CN. d Charge transfer and separation process happening in rGO/pCN nano-composite for CO2 reduction to CH4 in the occurrence of H2O under vis-light lighting.
In addition, formation method of 2D/2D structure may affect photo-catalytic activity; for example, exfoliation production of graphene and g-C3N4 will expose defects present on surface those are helpful for CO2 reduction, but surplus functional groups on catalyst surface will decrease conductance and number of active sites, therefore degrading activity. Therefore, Yu Teng Liang et al. [305] formed smaller quantity of surface defects that resulted in a higher photo-catalytic CO2 reduction activity toward CH4 formation as compared to their counterparts with high quantity of surface defects. In this method, graphene was formed by two different techniques that involved rGO solvent-exfoliated graphene (SEG) way and coupled with TiO2 to make rGO-TiO2 and SEG-TiO2, correspondingly. Due to its outstanding electron conductivity, SEG-TiO2 showed higher photo-catalytic CO2 reduction activity compared to rGO-TiO2. Hence, Table 2 shows the current developments in 2D materials for solar CO2 reduction devices.
7.4 Photo-catalytic H2O2 Production
Carbonaceous nanomaterials (NMs) with unique characteristics of sp2-hybridized carbon bonding with remarkable physicochemical nature at nanoscale usually show outstanding mechanical, chemical, and electrical properties [323]. In photo-catalysis research direction, carbonaceous NMs are frequently acted as e− transfer materials and photo-sensitizer that can widen adsorption edge and advance migration effectiveness of photo-induced e−s [324]. While carbonaceous NMs are immobilized on g-C3N4 photo-catalyst, they can accept and transport photo-induced e−s from CB level of g-C3N4 and boost the reduction reaction activity and so lead to superior photo-catalytic performance [325]. As typical carbonaceous NMs, CNTs with π-conjugative structure are able to accept, transport, and store e−s [326]. So, g-C3N4-CNTs fabricated by incorporating the CNTs into g-C3N4 can advance photo-catalytic activity. Zhao and co-authors [327] applied the amination method to initiate CNTs in g-C3N4 NSs to make hybrid catalyst of g-C3N4/CNTs, where CNTs were covalently mixed with g-C3N4. The g-C3N4/CNTs hybrid photo-catalyst showed H2O2 formation rate of 32.6 μmol h−1 that was noticeably higher than g-C3N4 (2.5 μmol h−1). The CNTs covalent combined with g-C3N4 advanced e− generation via higher reduction capability and favorably shifted CB level to improve single e− reduction of O2 to ·O2−.
When polyoxometalates (POMs) [328] are irradiated through plentiful light energy, excitation of charge transfer from O2− to Mn+ (n = 5, 6) is observed, guiding to development of h+ center (O−) and trapped e− center (M +(n−1) ) pair. So, excited POMs can provide e− donors/acceptors. In addition, POMs have lesser recombination possibility of e−s and h+s, as a result of its distinct HOMO–LUMO BGs. Taking advantages of these, POMs have been broadly used in photo-catalysts areas, for example H2O oxidation [329], H2-evolution [330], CO2-reduction [331], etc. Thus far, POMs also combined with g-C3N4 for photo-catalytic H2O2 evolution. Zhao and co-workers [332] combined POM cluster of [PW11O39]7-(PW11) with 3D ordered macro-porous g-C3N4 (3DOM g-C3N4) for efficient photo-catalytic H2O2 evolution. POM covalent cluster of PW11 was covalently bonded with 3DOM g-C3N4 by captivating an organic linker approach. The quantity of synthesized H2O2 by g-C3N4/PW11 attained 3.5 μmol within 60 min, whereas catalytic activity of pristine 3DOM g-C3N4 was only 1.3 μmol. The CB and VB of 3DOM g-C3N4/PW11 were more positive compared to 3DOM g-C3N4 that improved their potential for H2O oxidation and promoted 2e− reduction of O2 to H2O2. Similarly, they also studied a further POM cluster, [SiW11O39]8–(SiW11), to covalently combine with g-C3N4 [333].
Compared to PW11, the SiW11 possessed more negative CB level, helping 2e− reduction of O2 to H2O2. Under sunlight irradiation (AM 1.5), H2O2 photo-catalytic production of 15.2 μmol h−1 over hybrid g-C3N4/SiW11 photo-catalyst was attained. Except for direct combination of POMs with g-C3N4, POMs-derived metal oxides were also incorporated with g-C3N4 to make hybrid photo-catalysts. Since POMs-derived metal oxides can accept, transport, and store e−s, resulting hybrid photo-catalysts are able to improve photo-induced e−s generation and therefore increase performance of reduction reaction for H2O2 formation. Zhao and Zhao [334] studied g-C3N4/PWO hybrid photo-catalyst through calcination of g-C3N4 precursor and (NH4)3PW12O40 (NH4-PW12) (POMs precursor). The hybrid g-C3N4/PWO photo-catalyst showed competent photo-catalytic appearance for photo-catalytic H2O2 fabrication by vis-light in the absence of organic e− donor. In addition, a similar group [335] utilized another POMs-derived metal oxide to include g-C3N4. The g-C3N4/CoWO hybrid photo-catalyst was fabricated via calcination of 3-amino 1, 2, 4-triazole (3-AT) and (NH4)8Co2W12O42 (NH4-Co2W12). Under vis-light irradiation, H2O2 was rapidly generated over g-C3N4/CoWO and the amount of formed H2O2 was 18.7 μmol in 60 min, while individual g-C3N4 offered very lower photo-catalytic activity (< 0.1 μmol in 60 min). CoWO-incorporated g-C3N4 framework could produce more e− for O2 reduction, while negative shifts of CB level from g-C3N4 to g-C3N4-CoWO enhanced single e− reduction of O2 to ·O2−. Also, incorporated CoWO advanced the oxidation of ·O2− to 1O2 by h+ and formed 1O2 proceeded 2e− reduction to H2O2. Every one of these is related to enhanced photo-catalytic activity for H2O2 production over g-C3N4/CoWO hybrid photo-catalyst.
Organic counterparts have advantages of economic, simple formation and mechanical flexibility [336]. Mainly, organic photo-catalysts are capable to concentrate on faults of their inorganic counterparts, for example heavy metal with sensitive toxicity and restricted level of active sites. g-C3N4 were recognized as representative organic polymer SC photo-catalysts. Recently, other organic SCs were also employed as photo-catalysts, e.g., triazine and aromatic diimides. Aromatic diimides possess high e− mobility and stability that are significant class of n-type organic SCs and incorporated with g-C3N4 for photo-catalytic H2O2 evolution. Shiraishi et al. [337] included facile way to aromatic diimide (pyromellitic diimide, PDI) in g-C3N4 network by facile thermal condensation. These results showed that both CB and VB levels were developed into more positive via incorporation of PDI units in g-C3N4 due to high e− affinity of PDI. This detail exposed that g-C3N4/PDI photo-catalyst has superior ability for O2 reduction; therefore, H2O2 evolution from H2O and O2 was promoted. Additionally, efficient synthesis of 1,4-endoperoxide species on photoexcited g-C3N4/PDI suppressed 1e− reduction of O2 and 4e− reduction of O2, thus promoting selective two-e− reduction of O2 to H2O2.
Likewise, other categories of aromatic diimides were also incorporated with g-C3N4, for example biphenyl diimide (BDI) [338] and mellitic triimide (MTI) [339]. In pristine H2O with O2, both g-C3N4/BDI and g-C3N4/MTI catalysts successfully produced millimolar levels of H2O2. For further activity improvement, rGO is incorporated with g-C3N4/PDI catalyst [340] that takes advantage from 2D single-carbon monolayer property of rGO with high charge carrier mobility and high photochemical and thermal stability. The g-C3N4/PDI/rGOx nano-hybrids photo-catalyst was synthesized by hydrothermal–calcination process. The photo-catalytic reaction testing showed that g-C3N4/PDI/rGO0.05 produced the largest amount of H2O2 (29 μmol) under vis-light irradiation within 24 h, which was higher compared to g-C3N4 and g-C3N4/PDI. The SCC efficiency value of g-C3N4/PDI/rGO0.05 was up to 0.20% that was higher compared to other counterparts. In this photo-catalyst system, rGO not only encouraged efficient charge division but also increased selective 2e− O2 reduction. The activity of g-C3N4/PDI-rGO photo-catalyst could be further improved by introducing BN because of spatial separation of e− and h+ onto rGO and BN, correspondingly [341]. Yang et al. [342] fabricated perylene imides (PI) on g-C3N4 NSs to construct an all-solid-state Z-scheme hetero-junction. The hybrid g-C3N4/PI photo-catalyst with Z-scheme arrangement promoted spatial separation of charge carriers, where photo-induced e−s in PI recombined with h+s in g-C3N4, while remaining h+s and e−s were left on PI and g-C3N4, correspondingly. Consequently, more e−s from CB of g-C3N4 part reduced O2 to produce more H2O2, while h+s of g-C3N4/PI oxidized OH− to ·OH that also later reacted to generate H2O2. The shift of H2O2 production from single-channel to two-channel leads to significant enhancement in photo-catalytic H2O2 evolution. In another study, anthraquinone (AQ) was fastened on g-C3N4 surface that attains analogous roles as other organic SCs [343].
Fei Xue et al. [344] studied efficient photo-catalytic pure H2O2 splitting for simultaneous H2 and H2O2 fabrication. Photo-catalytic overall H2O splitting for instantaneous H2 and H2O2 generation via a 2e− pathway can readily address these issues. A novel CoxNiyP cluster incorporated P-doped g-C3N4 photo-catalyst (CoxNiyP-PCN) by two-step phosphating method that presents such unique behavior for pure H2O splitting into stoichiometric H2 and H2O2. The highest H2 production rate reaches 239.3 μmol h−1 g−1, achieved over CoNiP-PCN photo-catalyst that is among the best reported activities for overall H2O splitting. It is found that both P and cluster co-catalyst are critical to remarkably improved photo-catalytic activity. Specifically, P as a substitution of C in PCN introduces a positive charge center (P+), reinforcing chemical connection between PCN and CoNiP, in the form of P+–Pδ−–Coδ+/Niδ+. This unique bridging effect, together with extended light absorption by P doping and optimized surface redox potential by co-catalyst integration, stimulates efficient vectorial charge transfer between PCN and CoNiP and subsequent surface mass exchange. In contrast, this also shows that well-satisfied band structure of PCN can facilitate the 2e− reaction pathway, which not only has implication for potential use of CoNiP-PCN as potential photo-catalyst for solar H2 manufacture, and offers a new idea for pure H2O splitting in particulate system [344]. Table 3 offers intuitive summary of photo-catalytic-based H2O2 production.
8 Future Perspective and Challenges
In this review, a large number of experimental as well as theoretical research works related to photo-catalysts are discussed. A lot of confronts are required to be solved for incorporation of 2D p–n junctions in mass production electronic elements. Two most imperative confronts are scalable production of 2D p–n junctions and environment degradation of 2DMs. Despite significant growth in ultra-thin 2DMs for photo-catalysis, it is still suffering from large number of challenges in this direction. First, apart from extensively developed ultra-thin 2DMs, for instance OH, MOs, and sulfides for photo-catalytic process, other types of probable ultra-thin 2DMs with novel structure or electronic properties for photo-catalysis should be investigated, for instance layer oxy-halides (e.g., FeOBr and Bi4VO8Cl), thiophosphates (e.g., CoPS3), multi-metal chalcogenides (for instance Cu2ZnGeS4), or metal-free SCs (e.g., C3N and C2N). Specially, ultra-thin 2DMs by means of intrinsic non-vdWs’ layer structure have great potential in photo-catalysis, since plentiful surface atoms along dangling bonds can assist to construct outstanding chemical surroundings for supporting molecular reaction chemisorptions and enhance catalytic reaction dynamics. Second, there is an exceptional large-scale approach to yield ultra-thin 2D photo-catalysts with controlled thickness or crystal structures. The scalable manufacturing of ultra-thin 2D photo-catalysts has immense importance for possible commercial applications, and therefore more concentration should be given to cost-efficient scalable production approach. Third, atomic-scale thicknesses permit ultra-thin materials along simply adjusting electronic structures that show an important effect on photo-catalytic performance. In spite of defect engineering, element doping, etc., or other efficient approaches for instance, engineering or tensile strain of surface state should be modified to engineer electronic structure and therefore enormously promote photo-catalytic activity. Fourth, ultra-thin 2D arrangement offers a perfect material model to distinguish catalysts’ active site, additional types of tuning, and reaction centers quantity to fulfill definite catalytic process requirement.
8.1 Scalable Synthesis and Environmental Degradation
First major confront is concerned with scalable synthesis of engineered vdWs hetero-structures with well-controlled edges. Still if deterministic assigned methods are flourishing at experimental level, they are not suitable for commercial purposes. Growth methods, such as CVD growth, have already been proven proficient for the growth of high-quality 2DMs such as lateral and vertical hetero-structures at laboratory level. The VdWs epitaxial methods are even more promising for fabrication of high-quality 2D hetero-structures. Upscale of such growth methods is probable, and the upcoming years will realize the application of higher-quality devices. A second, potential approach is to upscale growth of 2D p–n junctions to merge development of single 2DMs (e.g., MoS2) with different doping methods (frequently electrostatic or chemical). A second confront is environment degradation of numerous recognized 2DMs. For instance, while exposed to air, the BP in its ultra-thin structure is likely to absorb humidity that degrades material electronic properties. In context of BP, the most established degradation mechanism such as material reaction with O2 alters material properties. One method that prevents this degradation is encapsulating air-sensitive material among h-BN flakes under O2− and humidity-free conditions. One active area of 2DMs investigation is consequently devoted to upscale encapsulation methods. A special move, which is at present practiced, is active investigation for novel 2DMs that did not create degradation problems, which could arrive either from the preparation (e.g., TiS3) or from natural resources (e.g., franckeite). Such, active search previously assisted to multiply number of present 2DMs only a handful more than twenty under 10 years.
8.2 Future Perspectives
Other than traditional optoelectronic applications, 2D p–n junctions have still a lot of unknown applications and fundamental problems. For example, thermoelectric applications of 2D p–n junctions were not yet completely studied. The conventional Peltier device, a component mainly utilized in electronics for cooling (and commonly less for heating), depends on p- and n-type SC thermally coupled in parallel and series. The VdWs hetero-structures could be utilized to manufacture atomic-level thin cooling (or heating) elements in combination with other smart properties of 2DMs, e.g., high transparency or flexibility. Another exciting way is the study of new p-n junction geometries (e.g., circular p-n junctions in recent times showed in G) or novel devices based on 2D p-n junctions, e.g., logic gates or memories. Genuine probabilities still buried in 2DMs are in all possibilities than one described and 2D p–n junctions grasp much promise in larger-scale applications. Such 2D-junctions are particularly attractive building blocks of inflexible and transparent electronics, e.g., light-emitting diodes (LEDs) or solar cells. One more application, which can advantage from ultra-thin structure of 2D p–n junctions, is light-sensing and harvesting applications for nano-photonics. The 2D p–n junctions can be utilized as photodetectors, and numerous material combinations are present, which can be utilized to propose devices with sensible wavelengths ranging from infrared to UV that have already been established.
9 Conclusion
In recent years, new p–n junctions manufacturing witnessed benefit of an ultra-thin nature of 2DMs. The top-down and bottom-up production methods have established competent generating p-n junctions with high optoelectronic properties. 2DMs continue to offer numerous prospects to manufacture new p-n junctions with exceptional properties, which unlock motivating scientific directions both in requisites of elementary questions and with respect to applications [32]. Ultra-thin 2DMs and their hybridizations through maintaining 2D arrangement are outstanding materials for elementary photo-catalytic investigation and promising marketable uses. This broad review highlights modern advancement in appliance of ultra-thin 2DMs for the photo-catalytic solar energy conversion. First, this review offers a complete outline of categorization and controlled fabrication mechanism of an ultra-thin 2D photo-catalyst. After that, approaches to modify electronic arrangement of ultra-thin2DMs and more effecting photo-catalytic properties are reviewed, that is, an element engineering, thickness engineering, defect engineering and doping. In addition, further hybridizations with upholding ultra-thin 2D characteristics are offered to further enhance photo-catalytic properties, such as QDs/2DMs, single atoms/2DMs, molecular/2DMs, and 2D–2D stacking materials. Lastly, a variety of photo-catalytic applications over ultra-thin 2D photo-catalysts were reviewed with emphasis on insights into structure–performance relationship, involving H2O oxidation, H2 evolution, CO2 reduction, N2 fixation, organic synthesis, and pollutants degradation. In conclusion, this review highlights universal approaches and current growth in 2D/2D hetero-junctions and hetero-structures that are outstanding candidates for basic investigation and possible catalyst applications because of their exclusive electronic structure and physicochemical properties:
-
1.
Integrating their components’ advantages, for instance ultra-thin 2D configuration, large surface area, and electronic/physicochemical properties;
-
2.
Partition or charge transfer can be encouraged for required function; and
-
3.
Versatile options (such as thickness, elements, defects, fabrication expertise, and contact space) can be designed to engineer properties and therefore application activity [136].
Heterogeneous photo-catalysis has turned into a fast-growing galaxy with manifold miscellaneous matters being investigated and introduced. Driven through aforementioned benefits and possible standards of 2D/2D hetero-structures, increasing amount of extraordinary achievements were established in last few years. However, with challenges creating hurdles for real catalysis applications, e.g., catalyst competency, selectivity related to yield and pollution, environment friendly, and cost-efficient, there are still a lot of efforts to follow the purpose of developing required catalyst and reaction scheme for real-world applications. Catalysts grasp key for increasing effectiveness of catalytic reaction method which is the entrance of commercial practicable applications required to be prevailed over. The 2D/2D hetero-structures as photo-catalysts are planned with purpose to endorse light harvesting, charge carrier separation/transfer, redox reactivity, etc. In addition to sufficiently intriguing benefits of 2D elements via very well engineering of structure, composition, BG, and surface reaction sites, interfacial tuning at nanoscale of 2D/2D hetero-structures is probable to further make easy photo-catalytic activity. Even though a lot of opportunities subsist for 2D/2D hetero-structures as electrocatalysts, this field is also facing a lot of challenges and research room:
-
1.
Synthetic techniques are further required to be optimized to persuade commercial manufacturing demands.
-
2.
Analysis techniques might be more superior for structure, physicochemical properties, and activity for these ultra-thin hetero-structures.
-
3.
Clear comprehension of work mechanism, in particular reaction intermediates, of 2D/2D hetero-junction electrocatalysts is still now required.
In addition, aforementioned optimization method of designing catalyst, high products yield/selectivity, and development of novel organic materials preparation are all solutions for further investigation. As a result of quick advancement and affluent information accumulated in the 2DMs and hetero-structures in last year’s, one may anticipate that 2D/2D hetero-structures would participate in a significant role in resolving energy and environment confronts [136]. However, investigations concentrating on the photo-catalysis mechanism are still insufficient. A lot of hard work should be required to optimize the theoretical calculation setting, nearer to the industrial-scale practical reaction conditions. Parallel exploitation of theoretical approach along superior experimental approach can assist to get deeper understanding for connection between neighboring atomic microstructure and activity as well as elucidate reaction methods. Also, due to relevant limitations of every material, a preferred move to obtain optimal properties is performed to assemble diverse building blocks to produce supportive results. It is supposed that such a broad review will further put research in the field of 2DMs as a novel photo-catalysis [32].
References
K. Huang, Z. Li, J. Lin, G. Han, P. Huang, Two-dimensional transition metal carbides and nitrides (mxenes) for biomedical applications. Chem. Soc. Rev. 47, 5109–5124 (2018). https://doi.org/10.1039/C7CS00838D
K. Khan, A.K. Tareen, M. Aslam, K.H. Thebo, U. Khan et al., A comprehensive review on synthesis of pristine and doped inorganic room temperature stable mayenite electride, [Ca24Al28O64]4+(e−)4 and its applications as a catalyst. Prog. Solid State Chem. 54, 1–19 (2018). https://doi.org/10.1016/j.progsolidstchem.2018.12.001
K. Khan, A.K. Tareen, M. Aslam, A. Mahmood, Q. Khan et al., Going green with batteries and supercapacitor: two dimensional materials and their nanocomposites based energy storage applications. Prog. Solid State Chem. 34, 100254 (2019). https://doi.org/10.1016/j.progsolidstchem.2019.100254
K. Khan, A.K. Tareen, M. Aslam, Y. Zhang, R. Wang, Z. Ouyang, Z. Gou, H. Zhang, Recent advances in two-dimensional materials and their nanocomposites in sustainable energy conversion applications. Nanoscale 11, 21622–21678 (2019). https://doi.org/10.1039/c9nr05919a
I. Dincer, Renewable energy and sustainable development: a crucial review. Renew. Sust. Energy Rev. 4, 157–175 (2000). https://doi.org/10.1016/S1364-0321(99)00011-8
X. Zou, Y. Zhang, Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 44, 5148–5180 (2015). https://doi.org/10.1039/C4CS00448E
X. Chia, A.Y.S. Eng, A. Ambrosi, S.M. Tan, M. Pumera, Electrochemistry of nanostructured layered transition-metal dichalcogenides. Chem. Rev. 115, 11941–11966 (2015). https://doi.org/10.1021/acs.chemrev.5b00287
H. Yin, Z. Tang, Ultrathin two-dimensional layered metal hydroxides: an emerging platform for advanced catalysis, energy conversion and storage. Chem. Soc. Rev. 45, 4873–4891 (2016). https://doi.org/10.1039/c6cs00343e
Y. Shi, H. Li, L.J. Li, Recent advances in controlled synthesis of two-dimensional transition metal dichalcogenides via vapour deposition techniques. Chem. Soc. Rev. 44, 2744–2756 (2015). https://doi.org/10.1039/C4CS00256C
Y. Jiao, Y. Zheng, M. Jaroniec, S.Z. Qiao, Design of electrocatalysts for oxygen-and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 44, 2060–2086 (2015). https://doi.org/10.1039/c4cs00470a
J. Pang, R.G. Mendes, A. Bachmatiuk, L. Zhao, H.Q. Ta et al., Applications of 2d mxenes in energy conversion and storage systems. Chem. Soc. Rev. 48, 72–133 (2019). https://doi.org/10.1039/C8CS00324F
H. Jin, C. Guo, X. Liu, J. Liu, A. Vasileff, Y. Jiao, Y. Zheng, S.Z. Qiao, Emerging two-dimensional nanomaterials for electrocatalysis. Chem. Rev. 118, 6337–6408 (2018). https://doi.org/10.1021/acs.chemrev.7b00689
Z. Zhang, E.S. Penev, B.I. Yakobson, Two-dimensional boron: structures, properties and applications. Chem. Soc. Rev. 46, 6746–6763 (2017)
Q. Meng, R.W. Xiu, J. Taeho, W. Miae, P.G. Young et al., Omnipotent phosphorene: a next-generation, two-dimensional nanoplatform for multidisciplinary biomedical applications. Chem. Soc. Rev. 47, 5588–5601 (2018). https://doi.org/10.1039/c8cs00342d
L. Dai, D.W. Chang, J.B. Baek, W. Lu, Carbon nanomaterials: carbon nanomaterials for advanced energy conversion and storage. Small 8, 1122 (2012). https://doi.org/10.1002/smll.201101594
K. Khan, L. Jia, Z. Wenwei, X. Wei, Y. Ye, S. Weijie, Low temperature synthesis of nano porous 12Cao·7Al2O3 powder by hydrothermal method. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 31, 1201–1205 (2016). https://doi.org/10.1007/s11595-016-1512-7
K. Khan, A.K. Tareen, S. Elshahat, A.K. Yadav, U. Khan et al., Facile synthesis of cationic doped [Ca24Al28O64]4+.(4e−) composite via rapid citrate sol–gel method. Dalton Trans. 47, 3819–3830 (2018). https://doi.org/10.1039/c7dt04543c
K. Khan, A.K. Tareen, J. Li, U. Khan, A. Nairan et al., Facile synthesis of tin-doped mayenite electride composite as a non-noble metal durable electrocatalyst for oxygen reduction reaction (ORR). Dalton Trans. 47, 13498–13506 (2018). https://doi.org/10.1039/c8dt02548g
K. Khan, A.K. Tareen, M. Aslam, R. Wang, Y. Zhang et al., Recent developments in emerging two-dimensional materials and their applications. J. Mater. Chem. C 9, 1–9 (2019). https://doi.org/10.1039/c9tc04187g
K. Khan, A.K. Tareen, M. Aslam, S.A. Khan, Q. Khan et al., Fe-doped mayenite electride composite with 2d reduced graphene oxide: as a non-platinum based, highly durable electrocatalyst for oxygen reduction reaction. Sci. Rep. 9, 19809 (2019). https://doi.org/10.1038/s41598-019-55207-6
K. Khan, A.K. Tareen, M. Aslam, Q. Khan, S.A. Khan et al., Novel two-dimensional carbon–chromium nitride-based composite as an electrocatalyst for oxygen reduction reaction. Front. Chem. 7, 738 (2019). https://doi.org/10.3389/fchem.2019.00738
K. Khan, A.K. Tareen, M. Aslam, R. Wang, Y. Zhang et al., Recent developments in emerging two-dimensional materials and their applications. J. Mater. Chem. C 8, 387–440 (2020). https://doi.org/10.1039/c9tc04187g
K. Khan, A.K. Tareen, S. Elshahat, N. Muhammad, J. Li et al., Facile metal-free reduction-based synthesis of pristine and cation-doped conductive mayenite. RSC Adv. 8, 24276–24285 (2018). https://doi.org/10.1039/c8ra02790krsc.li/rsc-advances
K. Khan, A.K. Tareen, U. Khan, A. Nairan, S. Elshahat et al., Single step synthesis of highly conductive room-temperature stable cation-substituted mayenite electride target and thin film. Sci. Rep. (2018). https://doi.org/10.1038/s41598-019-41512-7
A.K. Tareen, G.S. Priyanga, K. Khan, E. Pervaiz, T. Thomas, M. Yang, Nickel-based transition metal nitride electrocatalysts for the oxygen evolution reaction. Chemsuschem 12, 3941–3954 (2019). https://doi.org/10.1002/cssc.201900553
W. Zou, K. Khan, X. Zhao, C. Zhu, J. Huang et al., Direct fabrication of C12A7 electride target and room temperature deposition of thin films with low work function. Mater. Res. Express 4, 1–20 (2017). https://doi.org/10.1088/2053-1591/aa63c7
K. Khan, A.K. Tareen, M. Aslam, M.F. Khan, Z. Shi et al., Synthesis, properties and novel electrocatalytic applications of the 2-D borophene xenes. Prog. Solid State Chem. 58, 100283 (2020). https://doi.org/10.1016/j.progsolidstchem.2020.100283
W. Fan, Q. Zhang, Y. Wang, Semiconductor-based nanocomposites for photocatalytic H2 production and Co2 conversion. Phys. Chem. Chem. Phys. 15, 2632–2649 (2013). https://doi.org/10.1039/c2cp43524a
B. Luo, G. Liu, L. Wang, Recent advances in 2D materials for photocatalysis. Nanoscale 8, 6904–6920 (2016). https://doi.org/10.1039/c6nr00546b
W.J. Zhang, W.W. Li, X.G. Chen, Z.G. Hu, W. Liu et al., Phonon mode and phase transition behaviors of (1 − x)PbSc1/2Ta1/2O3−xPbHfO3 relaxor ferroelectric ceramics determined by temperature-dependent raman spectra. Appl. Phys. Lett. 99, 074103 (2011). https://doi.org/10.1063/1.3614431
R.D. Bonito, M.L. Elliott, E.A.D. Jardinm, Detection of an arbuscular mycorrhizal fungus in roots of different plant species with the PCR. Appl. Environ. Microb. 61, 2809–2810 (1995)
J. Di, J. Xiong, H. Li, Z. Liu, Ultrathin 2d photocatalysts: electronic-structure tailoring, hybridization, and applications. Adv. Mater. 30, 1–30 (2017). https://doi.org/10.1002/adma.201704548
J. Ran, J. Zhang, J. Yu, M. Jaroniec, S.Z. Qiao, Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 46, 7787–7812 (2015). https://doi.org/10.1039/C3CS60425J
Y. Ma, X. Wang, Y. Jia, X. Chen, H. Han, C. Li, Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 114, 9987–10043 (2014). https://doi.org/10.1021/cr500008u
L.T.C. Equally, J. Zhang, L.Z. Li, N.A. Kumar, X.S. Zhao, Functionalization of chemically derived graphene for improving its electrocapacitive energy storage properties. Energy Environ. Sci. 9, 1891–1930 (2016). https://doi.org/10.1039/C6EE00158K
W.J. Ong, L.L. Tan, Y.H. Ng, S.T. Yong, S.P. Chai, Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: are we a step closer to achieving sustainability? Chem. Rev. 116, 7159–7329 (2016). https://doi.org/10.1021/acs.chemrev.6b00075
A. Fujishima, K. Honda, Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972). https://doi.org/10.1038/238037a0
H.M. Tosine, J. Lawrence, J.H. Carey, Photodechlorination of PCB’s in the presence of titanium dioxide in aqueous suspensions. B-Environ. Contam. Toxicol. 16, 697–701 (1976). https://doi.org/10.1007/BF01685575
X. Zhou, N. Liu, J. Schmidt, A. Kahnt, A. Osvet et al., Noble-metal-free photocatalytic hydrogen evolution activity: the impact of ball milling anatase nanopowders with TiH2. Adv. Mater. 29, 1604747 (2017). https://doi.org/10.1002/adma.201604747
W.B. Choi, D.S. Chung, J.H. Kang, H.Y. Kim, Y.W. Jin et al., Fully sealed, high-brightness carbon-nanotube field-emission display. Appl. Phys. Lett. 75, 3129 (1999). https://doi.org/10.1063/1.125253
T. Hisatomi, K. Maeda, K. Takanabe, J. Kubota, K. Domen, Aspects of the water splitting mechanism on (Ga1 − xZnx)(n1 − xOx) photocatalyst modified with Rh2–YCrYo3 cocatalyst. J. Phys. Chem. C 113, 21458–21466 (2009). https://doi.org/10.1021/jp9079662
T. Hisatomi, J. Kubota, K. Domen, Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 43, 7520–7535 (2014). https://doi.org/10.1039/C3CS60378D
Q. Bao, H. Zhang, B. Wang, Z. Ni, C.H.Y.X. Lim et al., Broadband graphene polarizer. Nat. Photonics 5, 411–415 (2011). https://doi.org/10.1038/nphoton.2011.102
W. Tao, N. Kong, X.Y. Ji, Y.P. Zhang, A. Sharma et al., Emerging two-dimensional monoelemental materials (Xenes) for biomedical applications. Chem. Soc. Rev. 48, 2891–2912 (2019). https://doi.org/10.1039/C8CS00823J
C. Tan, X. Cao, X.J. Wu, Q. He, J. Yang et al., Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 117, 6225–6331 (2017). https://doi.org/10.1021/acs.chemrev.6b00558
K.S. Novoselov, A.K. Geim, S.V. Morozov, D. Jiang, Y. Zhang et al., Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004). https://doi.org/10.1126/science.1102896
Y. Sun, S. Gao, F. Lei, Y. Xie, Atomically-thin two-dimensional sheets for understanding active sites in catalysis. Chem. Soc. Rev. 44, 623–636 (2014). https://doi.org/10.1039/C4CS00236A
Z. Du, S. Yang, S. Li, J. Lou, S. Zhang et al., Conversion of non-van der waals solids to 2d transition-metal chalcogenides. Nature 577, 492–496 (2020). https://doi.org/10.1038/s41586-019-1904-x
T. Su, Q. Shao, Z. Qin, Z. Guo, Z. Wu, Role of interfaces in two-dimensional photocatalyst for water splitting. ACS Catal. 8, 2253–2276 (2018). https://doi.org/10.1021/acscatal.7b03437
J. Low, S. Cao, J. Yu, S. Wageh, Two-dimensional layered composite photocatalysts. Chem. Commun. 50, 10768–10777 (2014). https://doi.org/10.1039/C4CC02553A
S. Kouser, A. Thannikoth, U. Gupta, U.V. Waghmare, C.N.R. Rao, 2D-gas as a photocatalyst for water splitting to produce H2O2. Small 11, 4723–4730 (2015). https://doi.org/10.1002/smll.201501077
Z. Chao, L. Wang, L. Gang, Q.L. Gao, H.M. Cheng, Template-free synthesis of Ta3N5 nanorod arrays for efficient photoelectrochemical water splitting. Chem. Commun. 49, 3019–3021 (2013). https://doi.org/10.1039/C3CC40760H
N. Singh, G. Jabbour, U. Schwingenschlgl, Optical and photocatalytic properties of two-dimensional MoS2. Eur. Phys. J. B 85, 392–471 (2012). https://doi.org/10.1140/epjb/e2012-30449-7
Q. Li, X. Li, S. Wageh, A.A. Al-Ghamdi, J. Yu, CdS/graphene nanocomposite photocatalysts. Adv. Energy Mater. 5, 1500010 (2015). https://doi.org/10.1002/aenm.201500010
Y. Li, Y.L. Li, B. Sa, R. Ahuja, Review of two-dimensional materials for photocatalytic water splitting from a theoretical perspective. Catal. Sci. Technol. 7, 545–559 (2017). https://doi.org/10.1039/C6CY02178F
G.F. Chen, T.Y. Ma, Z.Q. Liu, N. Li, S.Z. Qiao, Efficient and stable bifunctional electrocatalysts Ni/Nix My (M = P, S) for overall water splitting. Adv. Funct. Mater. 26, 3314–3323 (2016). https://doi.org/10.1002/adfm.201505626
M. Monai, M. Melchionna, P. Fornasiero, Chapter One - From metal to metal-free catalysts: Routes to sustainable chemistry. Adv. Catal. 63, 1–73 (2018). https://doi.org/10.1016/bs.acat.2018.10.001
T. Tian, Y. Li, D. Xie, Y. Shen, J. Ren et al., Clinical features and risk factors for post-partum depression in a large cohort of chinese women with recurrent major depressive disorder. J. Affect. Disord. 136, 983–987 (2012). https://doi.org/10.1016/j.jad.2011.06.047
X. Li, M.W. Lin, J. Lin, B. Huang, A.A. Puretzky et al., Two-dimensional GaSe/MoSe2 misfit bilayer heterojunctions by van der waals epitaxy. Sci. Adv. 2, 1501882 (2016). https://doi.org/10.1126/sciadv.1501882
Q. Liang, L. Ye, Z.H. Huang, Q. Xu, Y. Bai, F. Kang, Q.H. Yang, A honeycomb-like porous carbon derived from pomelo peel for use in high-performance supercapacitors. Nanoscale 6, 13831–13837 (2014). https://doi.org/10.1039/c4nr04541f
R. Kobayashi, S. Tanigawa, T. Takashima, B. Ohtani, H. Irie, Silver-inserted heterojunction photocatalysts for z-scheme overall pure-water splitting under visible-light irradiation. J. Phys. Chem. C 118, 22450–22456 (2014). https://doi.org/10.1021/jp5069973
B. Lin, H. Li, H. An, W. Hao, J. Wei et al., Preparation of 2d/2d g-C3N4 nanosheet@ZNiN2S4 nanoleaf heterojunctions with well-designed high-speed charge transfer nanochannels towards high-efficiency photocatalytic hydrogen evolution. Appl. Catal. B Environ. 220, 542–552 (2018). https://doi.org/10.1016/j.apcatb.2017.08.071
H. Xiao, C. Tan, Z.Y.A. Zhang, 25th anniversary article: hybrid nanostructures based on two-dimensional nanomaterials. Adv. Mater. 26, 2185–2204 (2014). https://doi.org/10.1002/adma.201304964
Y.J. Wang, Y.M. Tao, F.Y. Li, Y.H. Wang, X.J. Xu et al., Pharmacological characterization of ATPM [(–)-3-aminothiazolo[5,4-b]-N-cyclopropylmethylmorphinan hydrochloride], a novel mixed κ-agonist and μ-agonist/-antagonist that attenuates morphine antinociceptive tolerance and heroin self-administration behavior. J. Pharmacol. Exp. Ther. 329, 306–313 (2009). https://doi.org/10.1124/jpet.108.142802
J. Yang, D. Wang, H. Han, C. Li, Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 46, 1900–1909 (2013). https://doi.org/10.1021/ar300227e
M. Melchionna, P. Fornasiero, Updates on the roadmap for photocatalysis. ACS Catal. 10, 5493–5501 (2020). https://doi.org/10.1021/acscatal.0c01204
B. Ohtani, Preparing articles on photocatalysis-beyond the illusions, misconceptions, and speculation. Chem. Lett. 37, 216–229 (2008). https://doi.org/10.1246/cl.2008.216
J. Zhang, Y. Huang, L. Jin, F. Rosei, F. Vetrone, J.P. Claverie, Efficient upconverting multiferroic core@shell photocatalysts: visible-to-near-infrared photon harvesting. ACS Appl. Mater. Interfaces. 9, 8142–8150 (2017). https://doi.org/10.1021/acsami.7b00158
L. Wang, X. Xu, Q. Cheng, S.X. Dou, Y. Du, Near-infrared-driven photocatalysts: design, construction, and applications. Small (2019). https://doi.org/10.1002/smll.201904107
M. Freitag, N. Möller, A. Rühling, C.A. Strassert, B.J. Ravoo, F. Glorius, Photocatalysis in the dark: near-infrared light driven photoredox catalysis by an upconversion nanoparticle/photocatalyst system. ChemPhotoChem 3, 24–27 (2019). https://doi.org/10.1002/cptc.201800212
H. Chen, W. Liu, B. Hu, Z. Qin, H. Liu, A full-spectrum photocatalyst with strong near-infrared photoactivity derived from synergy of nano-heterostructured Er3+-doped multi-phase oxides. Nanoscale 9, 18940–18950 (2017). https://doi.org/10.1039/C7NR08090E
S.E. Braslavsky, A.M. Braun, A.E. Cassano, A.V. Emeline, M.I. Litter et al., Glossary of terms used in photocatalysis and radiation catalysis. Pure Appl. Chem. 83, 931–1014 (2011). https://doi.org/10.1351/PAC-REC-09-09-36
M.J. Munoz-Batista, U. Caudillo-Flores, F. Ung-Medina, M. del Carmen Chávez-Parga, J.A. Cortés, A. Kubacka, M. Fernández-García, Gas phase 2-propanol degradation using titania photocatalysts: study of the quantum efficiency. Appl. Catal. B Environ. 201, 400–410 (2017). https://doi.org/10.1016/j.apcatb.2016.08.014
M. Shelef, R.W. McCabe, Twenty-five years after introduction of automotive catalysts: what next? Catal. Today 62, 35–50 (2000). https://doi.org/10.1016/S0920-5861(00)00407-7
S. Sui, X. Wang, X. Zhou, Y. Su, S. Riffat, C.-J. Liu, A comprehensive review of pt electrocatalysts for the oxygen reduction reaction: nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 5, 1808–1825 (2017). https://doi.org/10.1039/C6TA08580F
J. Kou, C. Lu, J. Wang, Y. Chen, Z. Xu, R.S. Varma, Selectivity enhancement in heterogeneous photocatalytic transformations. Chem. Rev. 117, 1445–1514 (2017). https://doi.org/10.1021/acs.chemrev.6b00396
G.J. Ruiz-Mercado, R.L. Smith, M.A. Gonzalez, Sustainability indicators for chemical processes: i. Taxonomy. Ind. Eng. Chem. Res. 51, 2309–2328 (2012). https://doi.org/10.1021/ie102116e
S.M. Fortier, N.T. Nassar, G.W. Lederer, J. Brainard, J. Gambogi, E.A. McCullough, Draft critical mineral list-summary of methodology and background information US geological survey technical input document in response to secretarial order no. 3359. 2018-1021 (2018). https://doi.org/10.3133/ofr20181021
A.L. Gulley, N.T. Nassar, S. Xun, China, the united states, and competition for resources that enable emerging technologies. Proc. Natl. Acad. Sci. U.S.A. 115, 4111–4115 (2018). https://doi.org/10.1073/pnas.1717152115
T. Paik, M. Cargnello, T.R. Gordon, S. Zhang, H. Yun et al., Photocatalytic hydrogen evolution from substoichiometric colloidal WO3−x nanowires. ACS Energy Lett. 3, 1904–1910 (2018). https://doi.org/10.1021/acsenergylett.8b00925
G. Carraro, C. Maccato, A. Gasparotto, T. Montini, S. Turner et al., Enhanced hydrogen production by photoreforming of renewable oxygenates through nanostructured Fe2O3 polymorphs. Adv. Funct. Mater. 24, 372–378 (2014). https://doi.org/10.1002/adfm.201302043
S. Qamar, F. Lei, L. Liang, S. Gao, K. Liu et al., Ultrathin TiO2 flakes optimizing solar light driven CO2 reduction. Nano Energy 26, 692–698 (2016). https://doi.org/10.1016/j.nanoen.2016.06.029
S. Gao, Y. Sun, F. Lei, J. Liu, L. Liang et al., Freestanding atomically-thin cuprous oxide sheets for improved visible-light photoelectrochemical water splitting. Nano Energy 8, 205–213 (2014). https://doi.org/10.1016/j.nanoen.2014.05.017
F. Lei, Y. Sun, K. Liu, S. Gao, L. Liang, B. Pan, Y. Xie, Oxygen vacancies confined in ultrathin indium oxide porous sheets for promoted visible-light water splitting. J. Am. Chem. Soc. 136, 6826–6829 (2014). https://doi.org/10.1021/ja501866r
J. Wang, C.J. Liu, Preparation of 2D WO3 nanomaterials with enhanced catalytic activities: current status and perspective. Chembioeng. Rev. 2, 335–350 (2015). https://doi.org/10.1002/cben.201500014
L. Wang, T. Sasaki, Titanium oxide nanosheets: graphene analogues with versatile functionalities. Chem. Rev. 114, 9455–9486 (2014). https://doi.org/10.1021/cr400627u
N. Sakai, Y. Ebina, K. Takada, T. Sasaki, Photocurrent generation from semiconducting manganese oxide nanosheets in response to visible light. J. Phys. Chem. B 109, 9651–9655 (2005). https://doi.org/10.1021/jp0500485
R. Ma, T. Sasaki, Nanosheets of oxides and hydroxides: ultimate 2d charge-bearing functional crystallites. Adv. Mater. 22, 5082–5104 (2011). https://doi.org/10.1002/adma.201001722
K. Akatsuka, G. Takanashi, Y. Ebina, M.A. Haga, T. Sasaki, Electronic band structure of exfoliated titanium- and/or niobium-based oxide nanosheets probed by electrochemical and photoelectrochemical measurements. J. Phys. Chem. C 116, 12426–12433 (2012). https://doi.org/10.1021/jp302417a
S. Ida, C. Ogata, M. Eguchi, W.J. Youngblood, T.E. Mallouk, Y. Matsumoto, Photoluminescence of perovskite nanosheets prepared by exfoliation of layered oxides, K2Ln2Ti3O10, KLnNb2o7, and RbLnTa2O7 (ln: lanthanide ion). J. Am. Chem. Soc. 39, 7052–7059 (2008). https://doi.org/10.1021/ja7114772
K. Maeda, M. Eguchi, T. Oshima, Perovskite oxide nanosheets with tunable band-edge potentials and high photocatalytic hydrogen-evolution activity. Angew. Chem. Int. Ed. 46, 13164 (2015). https://doi.org/10.1002/anie.201408441
H. Yu, Q. Sun, X. Jia, X. Wang, J. Yu, Facile synthesis of porous Bi2WO6 nanosheets with high photocatalytic performance. Dalton Trans. 44, 14532–14539 (2015). https://doi.org/10.1039/C5DT01859E
E.L. Tae, K.E. Lee, J.S. Jeong, K.B. Yoon, Synthesis of diamond-shape titanate molecular sheets with different sizes and realization of quantum confinement effect during dimensionality reduction from two to zero. J. Am. Chem. Soc. 130, 6534–6543 (2008). https://doi.org/10.1021/ja711467g
Y. Zhou, Y. Zhang, M. Lin, J. Long, Z. Zhang et al., Monolayered Bi2WO6 nanosheets mimicking heterojunction interface with open surfaces for photocatalysis. Nat. Commun. 6, 8340 (2015). https://doi.org/10.1038/ncomms9340
J. Li, S. Qin, J. Xu, J. Xiong, C. Wu et al., Randomized, double-blind, placebo-controlled phase iii trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J. Clin. Oncol. 34, 1448–1454 (2016). https://doi.org/10.1200/JCO.2015.63.5995
T. Zhang, C. Zhou, Y. Zhao, T. Bian, L. Shang et al., Bubble template synthesis of Sn2Nb2O7 hollow spheres for enhanced visible-light-driven photocatalytic hydrogen production. Chem. Commun. 49, 9872–9874 (2013). https://doi.org/10.1039/c3cc45683h
Y. Zhao, G. Chen, T. Bian, C. Zhou, T. Zhang, Defect-rich ultrathin ZnAl-layered double hydroxide nanosheets for efficient photoreduction of CO2 to CO with water. Adv. Mater. 27, 7823 (2015). https://doi.org/10.1002/adma.201503730
Y.R. Liu, E.W. Loh, T.H. Lan, S.F. Chen, Y.H. Yu et al., ADRA1A gene is associated with BMI in chronic schizophrenia patients exposed to antipsychotics. Pharmacogenomics J. 10, 30–39 (2010). https://doi.org/10.1038/tpj.2009.55
Y. Zhao, B. Li, Q. Wang, W. Gao, C.J. Wang et al., NiTi-layered double hydroxides nanosheets as efficient photocatalysts for oxygen evolution from water using visible light. Chem. Sci. 5, 951–958 (2014). https://doi.org/10.1039/C3SC52546E
A. Hasani, M. Tekalgne, Q.V. Le, H.W. Jang, S.Y. Kim, Two-dimensional materials as catalysts for solar fuels: hydrogen evolution reaction and CO2 reduction. J. Mater. Chem A 7, 430–454 (2019). https://doi.org/10.1039/C8TA09496A
Y. Xu, W. Zhao, R. Xu, Y. Shi, B. Zhang, Synthesis of ultrathin CdS nanosheets as efficient visible-light-driven water splitting photocatalysts for hydrogen evolution. Chem. Commun. 49, 9803–9805 (2013). https://doi.org/10.1039/C3CC46342G
Q. He, C. Li, F. Geng, H. Yang, P. Li et al., Aerosol optical properties retrieved from sun photometer measurements over Shanghai, China. J. Geophys. Res. Atmos. 117, 16204 (2012). https://doi.org/10.1029/2011JD017220
H. Li, J. Wu, Z. Yin, H. Zhang, Preparation and applications of mechanically exfoliated single-layer and multi layer MoS2 and WSe2 nanosheets. ACS Chem. Res. 47, 1067–1075 (2014). https://doi.org/10.1021/ar4002312
J. Yu, C.Y. Xu, F.X. Ma, S.P. Hu, L. Zhen, Monodisperse sns2 nanosheets for high-performance photocatalytic hydrogen generation. ACS Appl. Mater. Interfaces. 6, 22370–22377 (2014). https://doi.org/10.1021/am506396z
C. Lin, X. Zhu, J. Feng, C. Wu, Y. Xie, Hydrogen-incorporated TiS2 ultrathin nanosheets with ultrahigh conductivity for stamp-transferrable electrodes. J. Am. Chem. Soc. 135, 5144–5151 (2013). https://doi.org/10.1021/ja400041f
Y.H. Sang, Z.H. Zhao, M.W. Zhao, P. Hao, Y.H. Leng, H. Liu, From UV to near-infrared, WS2 nanosheet: a novel photocatalyst for full solar light spectrum photodegradation. Adv. Mater. 27, 363–369 (2015). https://doi.org/10.1002/adma.201403264
Y. Wu, M. Xu, X. Chen, S. Yang, H. Wu, J. Pan, X. Xiong, CTAB-assisted synthesis of novel ultrathin MoSe2 nanosheets perpendicular to graphene for adsorption and photodegradation of organic dyes under visible light. Nanoscale 8, 440–450 (2015). https://doi.org/10.1039/C5NR05748E
S. Balendhran, S. Walia, H. Nili, J.Z. Ou, S. Zhuiykov et al., Semiconductors: two-dimensional molybdenum trioxide and dichalcogenides. Adv. Funct. Mater. 23, 3946 (2013). https://doi.org/10.1002/adfm.201370159
M. Chhowalla, H.S. Shin, G. Eda, L.J. Li, K.P. Loh, H. Zhang, The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 5, 263–275 (2013). https://doi.org/10.1038/nchem.1589
J.T. Jang, S. Jeong, J.W. Seo, M.C. Kim, E. Sim et al., Ultrathin zirconium disulfide nanodiscs. J. Am. Chem. Soc. 133, 7636–7639 (2011). https://doi.org/10.1021/ja200400n
J. Xie, J. Zhang, L. Shuang, F. Grote, X. Zhang et al., Correction to controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. J. Am. Chem. Soc. 136, 1680 (2014). https://doi.org/10.1021/ja4129636
J. Hou, S. Cao, Y. Wu, F. Liang, L. Ye, Z. Lin, L. Sun, Perovskite-based nanocubes with simultaneously improved visible-light absorption and charge separation enabling efficient photocatalytic CO2 reduction. Nano Energy 30, 59–68 (2016). https://doi.org/10.1016/j.nanoen.2016.09.033
W.Q. Li, G. Wang, X.N. Zhang, H.P. Geng, J.L. Shen et al., Geometrical and morphological optimizations of plasmonic nanoarrays for high-performance SERS detection. Nanoscale (2015). https://doi.org/10.1039/C5NR03140K
M. Ablikim, S.S. Fang, H.X. Yang, M.G. Zhao, G.S. Varner et al., Observation of the decay ψ(2S) → k(892)k−+c.c. Phys. Lett. B 614, 752–769 (2005). https://doi.org/10.1016/j.physletb.2005.03.071
J. Di, J. Xia, Y. Huang, M. Ji, W. Fan, Z. Chen, H. Li, Constructing carbon quantum dots/Bi2SiO5 ultrathin nanosheets with enhanced photocatalytic activity and mechanism investigation. Chem. Eng. J. 302, 334–343 (2016). https://doi.org/10.1016/j.cej.2016.05.009
H. Liu, F. Wang, L. Liu, X.Y. Jia, W. Zheng et al., Synthesis, characterization, and ethylene polymerization behaviors of late-transition metal complexes coordinated with chlorinated bis(arylimino)pyridine ligand. Polymer 55, 4611–4618 (2014). https://doi.org/10.1016/j.polymer.2014.07.015
L. Tan, X.C. Zhu, M.S. Tan, L. Sun, L. Tan et al., The genetic variation of ARRB2 is associated with late-onset Alzheimer’s disease in Han Chinese. Curr. Alzheimer Res. 11, 408–412 (2014). https://doi.org/10.2174/1567205011666140317095014
A. Hameed, T. Montini, V. Gombac, P. Fornasiero, Surface phases and photocatalytic activity correlation of Bi2O3/Bi2O4-x nanocomposite. J. Am. Chem. Soc. 130, 9658–9659 (2008). https://doi.org/10.1021/ja803603y
M. Guan, C. Xiao, J. Zhang, S. Fan, Y. Xie, Vacancy associates promoting solar-driven photocatalytic activity of ultrathin bismuth oxychloride nanosheets. J. Am. Chem. Soc. 135, 10411–10417 (2013). https://doi.org/10.1021/ja402956f
J. Di, J.X. Xia, M.X. Ji, B. Wang, S. Yin et al., Advanced photocatalytic performance of graphene-like BN modified BIOBr flower-like materials for the removal of pollutants and mechanism insight. Appl. Catal. B Environ. 183, 254–262 (2016). https://doi.org/10.1016/j.apcatb.2015.10.036
D.R. Dreyer, S. Park, C.W. Bielawski, R.S. Ruoff, The chemistry of graphene oxide. Chem. Soc. Rev. 39, 228–240 (2010). https://doi.org/10.1039/b917103g
T.F. Yeh, J.M. Syu, C. Cheng, T.H. Chang, H. Teng, Graphite oxide as a photocatalyst for hydrogen production from water. Adv. Funct. Mater. 20, 2255–2262 (2010). https://doi.org/10.1002/adfm.201000274
J. Chu, J. Sun, L.I. Peng, L.I. Guangsheng, Y. Niu et al., Effect of platelet-rich plasma combined with human umbilical cord-mesenchymal stem cells on the healing of osteoporotic fracture in rats. Chin. J. Osteoporos. 135, 10411–10417 (2016). https://doi.org/10.1155/2016/9458396
Z. Zhou, J. Wang, J. Yu, Y. Shen, Y. Zhang, Dissolution and liquid crystals phase of 2D polymeric carbon nitride. J. Am. Chem. Soc. 137, 2179–2182 (2015). https://doi.org/10.1021/ja512179x
N. Ping, L. Zhang, G. Liu, H.M. Cheng, Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater. 2, 4763–4770 (2012). https://doi.org/10.1002/adfm.201200922
S. Yang, Y. Gong, J. Zhang, Z. Liang, P.M.A. Jayan, Exfoliated graphitic carbon nitride nanosheets as efficient catalysts for hydrogen evolution under visible light. Adv. Mater. 25, 2452–2456 (2013). https://doi.org/10.1002/adma.201204453
C.X. Liu, Z.Y. Luo, Y.W. Li, M. Chen, J. Xu et al., Active waveguides by low-fluence carbon implantation in Nd3+-doped fluorophosphate glasses. Mod. Phys. Lett. B 30, 1550266 (2016). https://doi.org/10.1142/S0217984915502668
H. Xu, J. Yan, X. She, L. Xu, J. Xia et al., Graphene-analogue carbon nitride: novel exfoliation synthesis and its application in photocatalysis and photoelectrochemical selective detection of trace amount of Cu2+. Nanoscale 6, 1406–1415 (2014). https://doi.org/10.1039/C3NR04759H
X. Zhang, X. Xie, H. Wang, J. Zhang, B. Pan, Y. Xie, Enhanced photoresponsive ultrathin graphitic-phase C3N4 nanosheets for bioimaging. J. Am. Chem. Soc. 135, 18–21 (2013). https://doi.org/10.1021/ja308249k
I. Hevesi, L. Nánai, R. Vajtai, Laser light stimulated oxidation of vanadium at nonuniform illumination. Superlattice Microstruct. 3, 409–412 (1987). https://doi.org/10.1016/0749-6036(87)90214-X
H. Ou, L. Lin, Y. Zheng, P. Yang, Y. Fang, X. Wang, Tri-s-triazine-based crystalline carbon nitride nanosheets for an improved hydrogen evolution. Adv. Mater. 29, 1700008 (2017). https://doi.org/10.1002/adma.201700008
Z.T.Z. Tao, K.W.K. Wang, F.Y.F. Yi, C.Y.C. Yan, Q.L.Q. Li et al., A 3D soc design for H.264 application with on-chip dram stacking, in 2010 IEEE International 3D Systems Integration Conference (3DIC), vol. 1, Corpus ID: 11735204 (2010). https://doi.org/10.1109/3DIC.2010.5751446
J. Ryu, Y.J. Jang, S. Choi, H.J. Kang, H. Park, J.S. Lee, S. Park, All-in-one synthesis of mesoporous silicon nanosheets from natural clay and their applicability to hydrogen evolution. NPG Asia Mater. 8, e248 (2016). https://doi.org/10.1038/am.2016.35
Y.P. Xie, J.-Y. Xing, X.-Y. Li, X. Wang, H.-J. Sun et al., Survey of sweetpotato viruses in China. Acta Virol. 57, 81–84 (2013). https://doi.org/10.4149/av_2013_01_81
O. Mashtalir, K.M. Cook, V.N. Mochalin, M. Crowe, M.W. Barsoum, Y. Gogotsi, Dye adsorption and decomposition on two-dimensional titanium carbide in aqueous media. J. Mater. Chem. A 2, 14334–14338 (2014). https://doi.org/10.1039/C4TA02638A
J. Su, G.D. Li, X.H. Li, J.S. Chen, 2D/2D heterojunctions for catalysis. Adv. Sci. 6, 1801702 (2019). https://doi.org/10.1002/advs.201801702
K. Maeda, M. Eguchi, T. Oshima, Perovskite oxide nanosheets with tunable band-edge potentials and high photocatalytic hydrogen-evolution activity. Angew. Chem. Int. Ed. 53, 13164–13168 (2014). https://doi.org/10.1002/anie.201408441
C.K. Ngaw, Q. Xu, T.T.Y. Tan, P. Hu, S. Cao, J.S.C. Loo, A strategy for in situ synthesis of well-defined core–shell Au@TiO2 hollow spheres for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 257, 112–121 (2014). https://doi.org/10.1016/j.cej.2014.07.059
J. Li, G. Zhan, Y. Yu, L. Zhang, Superior visible light hydrogen evolution of Janus bilayer junctions via atomic-level charge flow steering. Nat. Commun. 7, 11480 (2016). https://doi.org/10.1038/ncomms11480
Y. Wu, Z. Li, W. Ma, Y. Huang, L. Huo et al., PDT-S-T: a new polymer with optimized molecular conformation for controlled aggregation and π–π stacking and its application in efficient photovoltaic devices. Adv. Mater. 25, 3449–3455 (2013). https://doi.org/10.1002/adma.201301174
E.Q. Chen, X.Q. Song, Y.L. Wang, T.Y. Zhou, L. Bai et al., Construction of a highly-active, liver-specific transcriptional regulatory element through combination of the albumin promoter and α-fetoprotein enhancer. Plasmid 79, 87–88 (2011). https://doi.org/10.1016/j.plasmid.2010.11.006
S. Deckoff-Jones, J. Zhang, C.E. Petoukhoff, M.K.L. Man, S. Lei et al., Observing the interplay between surface and bulk optical nonlinearities in thin van der Waals crystals. Sci. Rep. 6, 22620 (2016). https://doi.org/10.1038/srep22620
Y. Sun, Z. Sun, S. Gao, H. Cheng, Q. Liu et al., Photoelectrochemical reactions: all-surface-atomic-metal chalcogenide sheets for high-efficiency visible-light photoelectrochemical water splitting. Adv. Energy Mater. 4, 1 (2014). https://doi.org/10.1002/aenm.201470002
Y. Sun, Z. Sun, S. Gao, H. Cheng, Q. Liu et al., All-surface-atomic-metal chalcogenide sheets for high-efficiency visible-light photoelectrochemical water splitting. Adv. Energy Mater. 4, 1300611 (2014). https://doi.org/10.1002/aenm.201300611
D. Liang, H. Luo, Y.F. Liu, Z.Y. Hao, Y. Wang et al., Lysilactones A–C, three 6H-dibenzo(b, d)pyran-6-one glycosides from Lysimachia clethroides, total synthesis of lysilactone A. Tetrahedron 69, 2093–2097 (2013). https://doi.org/10.1016/j.tet.2013.01.029
Y. Li, Z. Wang, T. Xia, H. Ju, K. Zhang et al., Implementing metal-to-ligand charge transfer in organic semiconductor for improved visible-near-infrared photocatalysis. Adv. Mater. 28, 6959–6965 (2016). https://doi.org/10.1002/adma.201601960
F. Lei, L. Zhang, Y. Sun, L. Liang, K. Liu et al., Atomic-layer-confined doping for atomic-level insights into visible-light water splitting. Angew. Chem. Int. Ed. 54, 9266–9270 (2015). https://doi.org/10.1002/ange.201503410
G. Liu, P. Niu, C.H. Sun, S.C. Smith, Z.G. Chen, G.Q. Lu, H.M. Cheng, Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4. J. Am. Chem. Soc. 132, 11642–11648 (2010). https://doi.org/10.1021/ja103798k
J. Zhou, Y. Huang, X. Cao, B. Ouyang, W. Sun et al., Two-dimensional NiCo2O4 nanosheet-coated three-dimensional graphene networks for high-rate, long-cycle-life supercapacitors. Nanoscale 7, 7035–7039 (2015). https://doi.org/10.1039/C4NR06527A
Q. Qiao, B.H. Li, C.X. Shan, J.S. Liu, J. Yu et al., Light-emitting diodes fabricated from small-size ZnO quantum dots. Mater. Lett. 74, 104–106 (2012). https://doi.org/10.1016/j.matlet.2012.01.048
C. Huang, C. Chen, M. Zhang, L. Lin, X. Ye et al., Carbon-doped BN nanosheets for metal-free photoredox catalysis. Nat. Commun. 6, 7698 (2015). https://doi.org/10.1038/ncomms8698
W. Bi, C. Ye, C. Xiao, W. Tong, X. Zhang, W. Shao, Y. Xie, Spatial location engineering of oxygen vacancies for optimized photocatalytic. Small 10, 2820–2825 (2014). https://doi.org/10.1002/smll.201303548
B.E.S. Collaboration, M. Ablikim, J.Z. Bai, Y. Ban, J.G. Bian et al., Observation of the decay (2S)k(892)k + c.c. Phys. Lett. B 614, 37–43 (2005). https://doi.org/10.1016/j.physletb.2005.03.071
S. Gao, B. Gu, X. Jiao, Y. Sun, X. Zu et al., Highly efficient and exceptionally durable CO2 photoreduction to methanol over freestanding defective single-unit-cell bismuth vanadate layers. J. Am. Chem. Soc. 139, 3438–3445 (2017). https://doi.org/10.1021/jacs.6b11263
M. Cargnello, T. Montini, S.Y. Smolin, J.B. Prieb, J.J. Delgado Jaén et al., Engineering titania nanostructure to tune and improve its photocatalytic activity. Proc. Natl. Acad. Sci. 113, 3966–3971 (2016). https://doi.org/10.1073/pnas.1524806113
J. Du, M. Zhang, Z. Guo, J. Chen, X. Zhu et al., Phosphorene quantum dot saturable absorbers for ultrafast fiber lasers. Sci. Rep. 7, 42357 (2017). https://doi.org/10.1038/srep42357
Y. Ge, Z. Zhu, Y. Xu, Y. Chen, S. Chen et al., Broadband nonlinear photoresponse of 2D TiS2 for ultrashort pulse generation and all-optical thresholding devices. Adv. Opt. Mater. 6, 1701166 (2018). https://doi.org/10.1002/adom.201701166
B. Guo, S.H. Wang, Z.X. Wu, Z.X. Wang, D.H. Wang et al., Sub-200 fs soliton mode-locked fiber laser based on bismuthene saturable absorber. Opt. Express 26, 22750 (2018). https://doi.org/10.1364/oe.26.022750
X. Jiang, S. Liu, W. Liang, S. Luo, Z. He et al., Broadband nonlinear photonics in few-layer MXene Ti3C2tx (t = F, O, or OH). Laser Photonics Rev. 12, 1700229–1700239 (2018). https://doi.org/10.1002/lpor.201700229
X. Jiang, L. Zhang, S. Liu, Y. Zhang, Z. He et al., Ultrathin metal–organic framework: an emerging broadband nonlinear optical material for ultrafast photonics. Adv. Opt. Mater. 6, 1800561 (2018). https://doi.org/10.1002/adom.201800561
Y. Song, Z. Liang, X. Jiang, Y. Chen, Z. Li et al., Few-layer antimonene decorated microfiber: ultra-short pulse generation and all-optical thresholding with enhanced long term stability. 2D Mater. 4, 045010 (2017). https://doi.org/10.1088/2053-1583/aa87c1
Z. Liu, H. Mu, S. Xiao, R. Wang, Z. Wang et al., Pulsed lasers employing solution-processed plasmonic Cu3−xP colloidal nanocrystals. Adv. Mater. 28, 3535–3542 (2016). https://doi.org/10.1002/adma.201504927
X. Zhu, S. Chen, M. Zhang, L. Chen, Q. Wu et al., TiS2-based saturable absorber for ultrafast fiber laser. Photonics Res. 6, C44 (2018). https://doi.org/10.1364/prj.6.000c44
P. Li, Y. Chen, T. Yang, Z. Wang, H. Lin et al., Two-dimensional CH3NH3PBi3 perovskite nanosheets for ultrafast pulsed fiber lasers. ACS Appl. Mater. Interfaces. 9, 12759–12765 (2017). https://doi.org/10.1021/acsami.7b01709
M. Zhang, Q. Wu, F. Zhang, L. Chen, X. Jin et al., 2D black phosphorus saturable absorbers for ultrafast photonics. Adv. Opt. Mater. 7, 1800224 (2018). https://doi.org/10.1002/adom.201800224
Y.F. Song, H. Zhang, D.Y. Tang, D.Y. Shen, Polarization rotation vector solitons in a graphene mode-locked fiber laser. Opt. Express 20, 27283–27289 (2012). https://doi.org/10.1364/OE.20.027283
Y. Chen, M. Wu, P. Tang, S. Chen, J. Du et al., The formation of various multi-soliton patterns and noise-like pulse in a fiber laser passively mode-locked by a topological insulator based saturable absorber. Laser Phys. Lett. 11, 055101 (2014). https://doi.org/10.1088/1612-2011/11/5/055101
Y. Ge, S. Chen, Y. Xu, Z. He, Z. Liang et al., Few-layer selenium-doped black phosphorus: synthesis, nonlinear optical properties and ultrafast photonics applications. J. Mater. Chem. C 5, 6129–6135 (2017). https://doi.org/10.1039/c7tc01267e
J. Li, H. Luo, B. Zhai, R. Lu, Z. Guo, H. Zhang, Y. Liu, Black phosphorus: a two-dimension saturable absorption material for mid-infrared q-switched and mode-locked fiber lasers. Sci. Rep. 6, 30361 (2016). https://doi.org/10.1038/srep30361
M. Liu, N. Zhao, H. Liu, X. Zheng, A. Luo et al., Dual-wavelength harmonically mode-locked fiber laser with topological insulator saturable absorber. IEEE Photonics Technol. Lett. 26, 983–986 (2014). https://doi.org/10.1109/lpt.2014.2311101
M. Liu, Z.R. Cai, S. Hu, A.P. Luo, C.J. Zhao et al., Dissipative rogue waves induced by long-range chaotic multi-pulse interactions in a fiber laser with a topological insulator-deposited microfiber photonic device. Opt. Lett. 40, 4767–4770 (2015). https://doi.org/10.1364/OL.40.004767
G. Zheng, Y. Chen, H. Huang, C. Zhao, S. Lu et al., Improved transfer quality of CVD-grown graphene by ultrasonic processing of target substrates: applications for ultra-fast laser photonics. ACS Appl. Mater. Interfaces. 5, 10288–10293 (2013). https://doi.org/10.1021/am403205v
Y.F. Song, H. Zhang, L.M. Zhao, D.Y. Shen, D.Y. Tang, Coexistence and interaction of vector and bound vector solitons in a dispersion-managed fiber laser mode locked by graphene. Opt. Express 24, 1814–1822 (2016). https://doi.org/10.1364/OE.24.001814
Y. Song, S. Chen, Q. Zhang, L. Li, L. Zhao, H. Zhang, D. Tang, Vector soliton fiber laser passively mode locked by few layer black phosphorus-based optical saturable absorber. Opt. Express 24, 25933–25942 (2016). https://doi.org/10.1364/OE.24.025933
Z.C. Luo, M. Liu, Z.N. Guo, X.F. Jiang, A.P. Luo et al., Microfiber-based few-layer black phosphorus saturable absorber for ultra-fast fiber laser. Opt. Express 23, 20030–20039 (2015). https://doi.org/10.1364/OE.23.020030
Q. Wang, Y. Chen, L. Miao, G. Jiang, S. Chen et al., Wide spectral and wavelength-tunable dissipative soliton fiber laser with topological insulator nano-sheets self-assembly films sandwiched by PMMA polymer. Opt. Express 23, 7681–7693 (2015). https://doi.org/10.1364/OE.23.007681
Y. Xu, W. Wang, Y. Ge, H. Guo, X. Zhang et al., Stabilization of black phosphorous quantum dots in PMMA nanofiber film and broadband nonlinear optics and ultrafast photonics application. Adv. Funct. Mater. 27, 1702437 (2017). https://doi.org/10.1002/adfm.201702437
X.F. Jiang, Z. Zeng, S. Li, Z. Guo, H. Zhang, F. Huang, Q.H. Xu, Tunable broadband nonlinear optical properties of black phosphorus quantum dots for femtosecond laser pulses. Materials (Basel) 10, 210 (2017). https://doi.org/10.3390/ma10020210
Z. Wang, Y. Xu, S.C. Dhanabalan, J. Sophia, C. Zhao et al., Black phosphorus quantum dots as an efficient saturable absorber for bound soliton operation in an erbium doped fiber laser. IEEE Photonics J. 8, 1–10 (2016). https://doi.org/10.1109/jphot.2016.2598085
C. Ma, C. Wang, B. Gao, J. Adams, G. Wu, H. Zhang, Recent progress in ultrafast lasers based on 2d materials as a saturable absorber. Appl. Phys. Rev. 6, 041304 (2019). https://doi.org/10.1063/1.5099188
T. Jiang, K. Yin, C. Wang, J. You, H. Ouyang et al., Ultrafast fiber lasers mode-locked by two-dimensional materials: review and prospect. Photonics Res. 8, 78 (2019). https://doi.org/10.1364/prj.8.000078
Y. Song, X. Shi, C. Wu, D. Tang, H. Zhang, Recent progress of study on optical solitons in fiber lasers. Appl. Phys. Rev. 6, 021313 (2019). https://doi.org/10.1063/1.5091811
Y. Fang, Y. Ge, C. Wang, H. Zhang, Mid-infrared photonics using 2D materials: status and challenges. Laser Photonics Rev. 14, 1900098 (2019). https://doi.org/10.1002/lpor.201900098
J. Zheng, X. Tang, Z. Yang, Z. Liang, Y. Chen et al., Few-layer phosphorene-decorated microfiber for all-optical thresholding and optical modulation. Adv. Opt. Mater. 5, 1700026 (2017). https://doi.org/10.1002/adom.201700026
J. Zheng, Z. Yang, C. Si, Z. Liang, X. Chen et al., Black phosphorus based all-optical-signal-processing: toward high performances and enhanced stability. ACS Photonics 4, 1466–1476 (2017). https://doi.org/10.1021/acsphotonics.7b00231
C. Wang, Y. Wang, X. Jiang, J. Xu, W. Huang et al., MXene Ti3C2Tx: a promising photothermal conversion material and application in all-optical modulation and all-optical information loading. Adv. Opt. Mater. 7, 1900060 (2019). https://doi.org/10.1002/adom.201900060
Y. Wang, W. Huang, J. Zhao, H. Huang, C. Wang et al., A bismuthene-based multifunctional all-optical phase and intensity modulator enabled by photothermal effect. J. Mater. Chem. C 7, 871–878 (2019). https://doi.org/10.1039/c8tc05513k
L. Wu, W. Huang, Y. Wang, J. Zhao, D. Ma et al., 2d tellurium based high-performance all-optical nonlinear photonic devices. Adv. Funct. Mater. 29, 1806346 (2019). https://doi.org/10.1002/adfm.201806346
S. Chen, L. Miao, X. Chen, Y. Chen, C. Zhao et al., Few-layer topological insulator for all-optical signal processing using the nonlinear kerr effect. Adv. Opt. Mater. 3, 1769–1778 (2015). https://doi.org/10.1002/adom.201500347
Y. Song, Y. Chen, X. Jiang, Y. Ge, Y. Wang et al., Nonlinear few-layer MXene-assisted all-optical wavelength conversion at telecommunication band. Adv. Opt. Mater. 7, 1801777 (2019). https://doi.org/10.1002/adom.201801777
Y. Wang, F. Zhang, X. Tang, X. Chen, Y. Chen et al., All-optical phosphorene phase modulator with enhanced stability under ambient conditions. Laser Photonics Rev. 12, 1800016 (2018). https://doi.org/10.1002/lpor.201800016
L. Wu, K. Chen, W. Huang, Z. Lin, J. Zhao et al., Perovskite CSPBX3: a promising nonlinear optical material and its applications for ambient all-optical switching with enhanced stability. Adv. Opt. Mater. 6, 1800400 (2018). https://doi.org/10.1002/adom.201800400
L. Wu, Y. Dong, J. Zhao, D. Ma, W. Huang et al., Kerr nonlinearity in 2D graphdiyne for passive photonic diodes. Adv. Mater. 31, 1807981 (2019). https://doi.org/10.1002/adma.201807981
L. Wu, X. Jiang, J. Zhao, W. Liang, Z. Li et al., Mxene-based nonlinear optical information converter for all-optical modulator and switcher. Laser Photonics Rev. 12, 1800215 (2018). https://doi.org/10.1002/lpor.201800215
L. Wu, Z. Xie, L. Lu, J. Zhao, Y. Wang et al., Few-layer tin sulfide: a promising black-phosphorus-analogue 2D material with exceptionally large nonlinear optical response, high stability, and applications in all-optical switching and wavelength conversion. Adv. Opt. Mater. 6, 1700985 (2018). https://doi.org/10.1002/adom.201700985
Q. Wu, S. Chen, Y. Wang, L. Wu, X. Jiang et al., MZI-based all-optical modulator using mxene Ti3C2Tx (T = F, O, or OH) deposited microfiber. Adv. Mater. Technol. 4, 1800532 (2019). https://doi.org/10.1002/admt.201800532
Y. Wang, W. Huang, C. Wang, J. Guo, F. Zhang et al., An all-optical, actively q-switched fiber laser by an antimonene-based optical modulator. Laser Photonics Rev. 13, 1800313 (2019). https://doi.org/10.1002/lpor.201800313
Q. Ou, Y. Zhang, Z. Wang, J.A. Yuwono, R. Wang et al., Strong depletion in hybrid perovskite p–n junctions induced by local electronic doping. Adv. Mater. 30, 1705792 (2018). https://doi.org/10.1002/adma.201705792
P. Guo, J. Xu, K. Gong, X. Shen, Y. Lu et al., On-nanowire axial heterojunction design for high-performance photodetectors. ACS Nano 10, 8474–8481 (2016). https://doi.org/10.1021/acsnano.6b03458
Z. Guo, S. Chen, Z. Wang, Z. Yang, F. Liu et al., Metal-ion-modified black phosphorus with enhanced stability and transistor performance. Adv. Mater. 29, 1703811 (2017). https://doi.org/10.1002/adma.201703811
Z. Huang, W. Han, H. Tang, L. Ren, D.S. Chander, X. Qi, H. Zhang, Photoelectrochemical-type sunlight photodetector based on MoS2/graphene heterostructure. 2D Mater. 2, 035011 (2015). https://doi.org/10.1088/2053-1583/2/3/035011
X. Ren, Z. Li, Z. Huang, D. Sang, H. Qiao et al., Environmentally robust black phosphorus nanosheets in solution: application for self-powered photodetector. Adv. Funct. Mater. 27, 1606834 (2017). https://doi.org/10.1002/adfm.201606834
Y. Xu, J. Yuan, K. Zhang, Y. Hou, Q. Sun et al., Field-induced n-doping of black phosphorus for CMOS compatible 2D logic electronics with high electron mobility. Adv. Funct. Mater. 27, 1702211 (2017). https://doi.org/10.1002/adfm.201702211
X. Ji, N. Kong, J. Wang, W. Li, Y. Xiao et al., A novel top-down synthesis of ultrathin 2D boron nanosheets for multimodal imaging-guided cancer therapy. Adv. Mater. 30, 1803031 (2018). https://doi.org/10.1002/adma.201803031
X. Liang, X. Ye, C. Wang, C. Xing, Q. Miao et al., Photothermal cancer immunotherapy by erythrocyte membrane-coated black phosphorus formulation. J. Control. Release 296, 150–161 (2019). https://doi.org/10.1016/j.jconrel.2019.01.027
M. Luo, T. Fan, Y. Zhou, H. Zhang, L. Mei, 2D black phosphorus-based biomedical applications. Adv. Funct. Mater. 29(13), 1808306 (2019). https://doi.org/10.1002/adfm.201808306
Z.B. Sun, Y.T. Zhao, Z.B. Li, H.D. Cui, Y.Y. Zhou et al., TiL4-coordinated black phosphorus quantum dots as an efficient contrast agent for in vivo photoacoustic imaging of cancer. Small 13, 1602896 (2017). https://doi.org/10.1002/smll.201602896
M. Qiu, D. Wang, W. Liang, L. Liu, Y. Zhang et al., Novel concept of the smart NIR-light-controlled drug release of black phosphorus nanostructure for cancer therapy. Proc. Natl. Acad. Sci. 115, 501–506 (2018). https://doi.org/10.1073/pnas.1714421115
F. Yin, K. Hu, S. Chen, D. Wang, J. Zhang et al., Black phosphorus quantum dot based novel siRNA delivery systems in human pluripotent teratoma PA-1 cells. J. Mater. Chem. B 5, 5433–5440 (2017). https://doi.org/10.1039/c7tb01068k
T. Fan, Y. Zhou, M. Qiu, H. Zhang, Black phosphorus: a novel nanoplatform with potential in the field of bio-photonic nanomedicine. J. Innov. Opt. Health Sci. 11, 1830003 (2018). https://doi.org/10.1142/s1793545818300033
W. Tao, X. Ji, X. Xu, M.A. Islam, Z. Li et al., Antimonene quantum dots: synthesis and application as near-infrared photothermal agents for effective cancer therapy. Angew. Chem. Int. Ed. 56, 11896–11900 (2017). https://doi.org/10.1002/anie.201703657
T. Xue, W. Liang, Y. Li, Y. Sun, Y. Xiang et al., Ultrasensitive detection of miRNA with an antimonene-based surface plasmon resonance sensor. Nat. Commun. 10, 28 (2019). https://doi.org/10.1038/s41467-018-07947-8
H. Xie, Z. Li, Z. Sun, J. Shao, X.F. Yu et al., Metabolizable ultrathin Bi2Se3 nanosheets in imaging-guided photothermal therapy. Small 12, 4136–4145 (2016). https://doi.org/10.1002/smll.201601050
W. Tao, X. Ji, X. Zhu, L. Li, J. Wang et al., Two-dimensional antimonene-based photonic nanomedicine for cancer theranostics. Adv. Mater. 30, 1802061 (2018). https://doi.org/10.1002/adma.201802061
J. Liu, Y. Liu, N. Liu, Y. Han, X. Zhang et al., Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 347, 970–974 (2015). https://doi.org/10.1126/science.aaa3145
J. Xia, J. Di, H. Li, H. Xu, H. Li, S. Guo, Ionic liquid-induced strategy for carbon quantum dots/BiOx (x = Br, Cl) hybrid nanosheets with superior visible light-driven photocatalysis. Appl. Catal. B Environ. 181, 260–269 (2016). https://doi.org/10.1016/j.apcatb.2015.07.035
F. Xiang, S. Nan, Y. Liu, X. Chen, X. Zhou, Simultaneously enhanced stability and selectivity for propene epoxidation with H2 and O2 on Au catalysts supported on nano-crystalline mesoporous TS-1. ACS Catal. 7, 2668–2675 (2017). https://doi.org/10.1021/acscatal.6b03498
B. Qiao, A. Wang, X. Yang, L.F. Allard, Z. Jiang et al., Single-atom catalysis of Co oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011). https://doi.org/10.1038/nchem.1095
Z. Chen, S. Pronkin, T.P. Fellinger, K. Kailasam, G. Vilé et al., Merging single-atom-dispersed silver and carbon nitride to a joint electronic system via copolymerization with silver tricyanomethanide. ACS Nano 10, 3166–3175 (2016). https://doi.org/10.1021/acsnano.5b04210
X. Li, W. Bi, L. Zhang, S. Tao, Y. Xie, Single-atom Pt as co-catalyst for enhanced photocatalytic H2 evolution. Adv. Mater. 28, 2427–2431 (2016). https://doi.org/10.1002/adma.201505281
S. Ida, N. Kim, E. Ertekin, S. Takenaka, T. Ishihara, Photocatalytic reaction centers in two-dimensional titanium oxide crystals. J. Am. Chem. Soc. 137, 239–244 (2014). https://doi.org/10.1021/ja509970z
Z. Han, F. Qiu, R. Eisenberg, P.L. Holland, T.D. Krauss, Robust photogeneration of H2 in water using semiconductor nanocrystals and a nickel catalyst. Science 338, 1321–1324 (2012). https://doi.org/10.1021/acscatal.5b02036
X. Lu, K. Xu, S. Tao, Z. Shao, X. Peng et al., Engineering the electronic structure of two-dimensional subnanopore nanosheets using molecular titanium-oxide incorporation for enhanced photocatalytic activity. Chem. Sci. 7, 1462–1467 (2016). https://doi.org/10.1039/C5SC03551A
Y.J. Yuan, Z.J. Ye, H. Lu, B. Hu, Y.H. Li et al., Constructing anatase TiO2 nanosheets with exposed (001) facets/layered MoS2 two-dimensional nanojunction for enhanced solar hydrogen generation. ACS Catal. 6, 532–541 (2016). https://doi.org/10.1021/acscatal.5b02036
S. Ida, A. Takashiba, S. Koga, H. Hagiwara, T. Ishihara, Potential gradient and photocatalytic activity of an ultrathin p–n junction surface prepared with two-dimensional semiconducting nanocrystals. J. Am. Chem. Soc. 136, 1872–1878 (2014). https://doi.org/10.1021/ja409465k
Y.Z. Huang, L.M. Wu, X.T. Wu, L.H. Li, L. Chen, Y.F. Zhang, Pb2B5O9I: an iodide borate with strong second harmonic generation. J. Am. Chem. Soc. 132, 12788–12789 (2010). https://doi.org/10.1021/ja106066k
Y. Hou, A.B. Laursen, J. Zhang, G. Zhang, Y. Zhu et al., Layered nanojunctions for hydrogen-evolution catalysis. Angew. Chem. Int. Ed. 125, 3709–3713 (2013). https://doi.org/10.1002/anie.201210294
Y.H. Huang, J.J. Wang, Z.M. Liu, G.D. Lin, H.B. Zhang, Highly efficient Ni–ZrO2 catalyst doped with YB2O3 for co-methanation of CO and CO2. Appl. Catal. A Gen. 466, 300–306 (2013). https://doi.org/10.1016/j.apcata.2013.06.021
F. Wen, C. An, X. Wu, Y. Yang, J. Xu et al., MiR-34a regulates mitochondrial content and fat ectopic deposition induced by resistin through the AMPK/PPARα pathway in HepG2 cells. Int. J. Biochem. Cell Biol. 94, 133–145 (2018). https://doi.org/10.1016/j.biocel.2017.11.008
J.L. Gunjakar, T.W. Kim, H.N. Kim, I.Y. Kim, S.J. Hwang, Mesoporous layer-by-layer ordered nanohybrids of layered double hydroxide and layered metal oxide: highly active visible light photocatalysts with improved chemical stability. J. Am. Chem. Soc. 133, 14998–15007 (2011). https://doi.org/10.1021/ja203388r
Y. Sun, Z. Sun, S. Gao, H. Cheng, Q. Liu et al., Fabrication of flexible and freestanding zinc chalcogenide single layers. Nat. Commun. 3, 1057 (2012). https://doi.org/10.1038/ncomms2066
Y. Liu, L. Liang, C. Xiao, X. Hua, Z. Li, B. Pan, Y. Xie, Promoting photogenerated holes utilization in pore-rich WO3 ultrathin nanosheets for efficient oxygen-evolving photoanode. Adv. Energy Mater. 6, 1600437 (2016). https://doi.org/10.1002/aenm.201600437
Y. Sun, Z. Sun, S. Gao, H. Cheng, Q. Liu et al., Fabrication of flexible and freestanding zinc chalcogenide single layers. Nat. Commun. 3, 1–7 (2012). https://doi.org/10.1038/ncomms2066
Q. He, C. Li, F. Geng, H. Yang, P. Li et al., Aerosol optical properties retrieved from sun photometer measurements over Shanghai, China. J. Geophys. Res. Atmos. 117, 74–82 (2012). https://doi.org/10.1029/2011JD017220
Y. Sun, Z. Sun, S. Gao, H. Cheng, Q. Liu et al., Photoelectrochemical reactions: all-surface-atomic-metal chalcogenide sheets for high-efficiency visible-light photoelectrochemical water splitting. Adv. Energy Mater. 4, 1300574 (2014). https://doi.org/10.1002/aenm.201470002
J. Zhu, Z. Yin, Y. Dan, T. Sun, Q. Yan, Hierarchical hollow spheres composed of ultrathin Fe2O3 nanosheets for lithium storage and photocatalytic water oxidation. Energy Environ. Sci. 6, 987–993 (2013). https://doi.org/10.1039/C2EE24148J
M. Ablikim, J.Z. Bai, Y. Bai, Y. Ban, X. Cai et al., Measurements of the observed cross sections for e+e− → exclusive light hadrons containing π0π0 at √s = 3.773, 3.650 and 3.6648 GeV. Phys. Lett. B 670, 179–183 (2008). https://doi.org/10.1016/j.physletb.2008.10.051
K.C. Kwon, S. Choi, K. Hong, D.M. Andoshe, J.M. Suh et al., Tungsten disulfide thin film/p-type si heterojunction photocathode for efficient photochemical hydrogen production. MRS Commun. 7, 272–279 (2017). https://doi.org/10.1002/anie.201210294
M. Zhang, J. Guan, Y. Tu, S. Chen, Y. Wang et al., Highly efficient H2 production from H2S via a robust graphene-encapsulated metal catalyst. Energy Environ. Sci. 13, 119–126 (2020). https://doi.org/10.1039/C9EE03231B
H. Wang, R. Peng, Z.D. Hood, M. Naguib, S.P. Adhikari, Z. Wu, Titania composites with 2D transition metal carbides as photocatalysts for hydrogen production under visible-light irradiation. Chemsuschem 9, 1490–1497 (2016). https://doi.org/10.1002/cssc.201600165
J. Peng, X. Chen, W.J. Ong, X. Zhao, N. Li, Surface and heterointerface engineering of 2D MXenes and their nanocomposites: insights into electro- and photocatalysis. Chem 5, 18–50 (2019). https://doi.org/10.1016/j.chempr.2018.08.037
T. Su, R. Peng, Z.D. Hood, M. Naguib, I.N. Ivanov, J.K. Keum, Z. Qin, Z. Guo, Z. Wu, One-step synthesis of Nb2O5/C/Nb2C (MXene) composites and their use as photocatalysts for hydrogen evolution. Chemsuschem 11, 688 (2018). https://doi.org/10.1002/cssc.201702317
J. Ran, G. Gao, F.T. Li, T.Y. Ma, A. Du, S.Z. Qiao, Ti3C2 Mxene co-catalyst on metal sulfide photo-absorbers for enhanced visible-light photocatalytic hydrogen production. Nat. Commun. 8, 13907 (2017). https://doi.org/10.1038/ncomms13907
M. Shao, Y. Shao, J.W. Chai, Y. Qu, H. Pan, Synergistic effect of 2D Ti2C and g-C3N4 for efficient photocatalytic hydrogen production. J. Mater. Chem. A 5, 16748–16756 (2017). https://doi.org/10.1039/C7TA04122E
A. Vasileff, C. Xu, Y. Jiao, Y. Zheng, S.Z. Qiao, Surface and interface engineering in copper-based bimetallic materials for selective CO2 electroreduction. Chem 4, 1809–1831 (2018). https://doi.org/10.1016/j.chempr.2018.05.001
L. Liang, F. Lei, S. Gao, Y. Sun, X. Jiao et al., Single unit cell bismuth tungstate layers realizing robust solar CO2 reduction to methanol. Angew. Chem. Int. Ed. 127, 14177–14180 (2015). https://doi.org/10.1002/anie.201506966
S. Asif, R. Kashif, N. Mohsin, M. Waheed, J. Jiseon et al., Heterostructural TiO2/Ti3C2Tx (MXene) for photocatalytic degradation of antiepileptic drug carbamazepine. Chem. Eng. J. 349, 748–755 (2017). https://doi.org/10.1021/jacs.6b11263
N. Li, X. Chen, W.J. Ong, D.R. MacFarlane, X. Zhao, A.K. Cheetham, C. Sun, Understanding of electrochemical mechanisms for CO2 capture and conversion into hydrocarbon fuels in transition-metal carbides (MXenes). ACS Nano 11, 10825–10833 (2017). https://doi.org/10.1021/acsnano.7b03738
Y. Bai, L. Ye, T. Chen, L. Wang, X. Shi, X. Zhang, D. Chen, Facet-dependent photocatalytic N2 fixation of bismuth-rich Bi5O7i nanosheets. ACS Appl. Mater. Interfaces. 8, 27661–27668 (2016). https://doi.org/10.1021/acsami.6b08129
H. Li, J. Shang, Z. Ai, L. Zhang, Efficient visible light nitrogen fixation with BIOBr nanosheets of oxygen vacancies on the exposed 001 facets. J. Am. Chem. Soc. 137, 6393–6399 (2015). https://doi.org/10.1021/jacs.5b03105
S. Cao, B. Shen, T. Tong, J. Fu, J. Yu, 2D/2D heterojunction of ultrathin mxene/Bi2Wo6 nanosheets for improved photocatalytic CO2 reduction. Adv. Funct. Mater. 28, 1800136 (2018). https://doi.org/10.1002/adfm.201800136
M. Ye, X. Wang, E. Liu, J. Ye, D. Wang, Boosting the photocatalytic activity of P25 for carbon dioxide reduction using a surface-alkalinized titanium carbide MXene as co-catalyst. Chemsuschem 11, 1606–1611 (2018). https://doi.org/10.1002/cssc.201800083
C. Liu, Q. Xu, Q. Zhang, Y. Zhu, J. Xu, Layered BioBr/Ti3C2 MXene composite with improved visible-light photocatalytic activity. J. Mater. Sci. 54, 2458–2471 (2019). https://doi.org/10.1007/s10853-018-2990-0
X.Y. Kong, W.L. Tan, B.J. Ng, S.P. Chai, A.R. Mohamed, Harnessing Vis-NIR broad spectrum for photocatalytic CO2 reduction over carbon quantum dots-decorated ultrathin Bi2Wo6 nanosheets. Nano Res. 10, 1720–1731 (2017). https://doi.org/10.1007/s12274-017-1435-4
J.R. Christianson, D. Zhu, R.J. Hamers, J.R. Schmidt, Mechanism of N2 reduction to NH3 by aqueous solvated electrons. J. Phys. Chem. B 118, 195–203 (2014). https://doi.org/10.1021/jp406535p
H. Li, F. Qin, Z. Yang, X. Cui, J. Wang, L. Zhang, New reaction pathway induced by plasmon for selective benzyl alcohol oxidation on BioCl possessing oxygen vacancies. J. Am. Chem. Soc. 139, 3513–3521 (2017). https://doi.org/10.1021/jacs.6b12850
N. Zhang, X. Li, H. Ye, S. Chen, H. Ju et al., Oxide defect engineering enables to couple solar energy into oxygen activation. J. Am. Chem. Soc. 138, 8928–8935 (2016). https://doi.org/10.1021/jacs.6b04629
X.B. Li, C.H. Liu, R. Zhang, X.T. Huang, Y.Y. Li et al., Determination and pharmacokinetics of amygdalin in rats by LC-MS–MS. J. Chromatogr. Sci. 52, 476–481 (2013). https://doi.org/10.1093/chromsci/bmt063
C. Peng, X. Yang, Y. Li, H. Yu, H. Wang, F. Peng, Hybrids of two-dimensional Ti3C2 and TiO2 exposing 001 facets toward enhanced photocatalytic activity. ACS Appl. Mater. Interfaces. 8, 6051–6060 (2016). https://doi.org/10.1021/acsami.5b11973
Z. Lin, D. Barbara, P.L. Taberna, K.L. Van Aken, B. Anasori, Y. Gogotsi, P. Simon, Capacitance of Ti3C2tx mxene in ionic liquid electrolyte. J. Power Sources 326, 575–579 (2016). https://doi.org/10.1016/j.jpowsour.2016.04.035
X. Xie, N. Zhang, Z.R. Tang, M. Anpo, Y.J. Xu, Ti3C2Tx MXene as a janus cocatalyst for concurrent promoted photoactivity and inhibited photocorrosion. Appl. Catal. B Environ. 237, 43–49 (2018). https://doi.org/10.1016/j.apcatb.2018.05.070
H. Wang, Y. Wu, T. Xiao, X. Yuan, G. Zeng et al., Formation of quasi-core–shell In2S3/anatase TiO2 @metallic Ti3C2tx hybrids with favorable charge transfer channels for excellent visible-light-photocatalytic performance. Appl. Catal. B Environ. 233, 213–225 (2018). https://doi.org/10.1016/j.apcatb.2018.04.012
J.M. Campos-Martin, G. Blanco-Brieva, J.L. Fierro, Hydrogen peroxide synthesis: an outlook beyond the anthraquinone process. Angew. Chem. Int. Ed. 45, 6962–6984 (2006). https://doi.org/10.1002/anie.200503779
K. Sato, M. Aoki, R. Noyori, A “Green” Route to adipic acid: direct oxidation of cyclohexenes with 30 percent hydrogen peroxide. Science 281, 1646–1647 (1998). https://doi.org/10.1126/science.281.5383.1646
S. Yang, A. Verdaguer-Casadevall, L. Arnarson, L. Silvioli, V. Čolić et al., Toward the decentralized electrochemical production of H2O2: a focus on the catalysis. ACS Catal. 8, 4064–4081 (2018). https://doi.org/10.1021/acscatal.8b00217
W. Zhan, L. Ji, Z.M. Ge, X. Wang, R.T. Li, A continuous-flow synthesis of primary amides from hydrolysis of nitriles using hydrogen peroxide as oxidant. Tetrahedron 74, 1527–1532 (2018). https://doi.org/10.1016/j.tet.2018.02.017
M. Ksibi, Chemical oxidation with hydrogen peroxide for domestic wastewater treatment. Chem. Eng. J. 119, 161–165 (2006). https://doi.org/10.1016/j.cej.2006.03.022
R.N. Gurram, M. Al-Shannag, N.J. Lecher, S.M. Duncan, E.L. Singsaas, M. Alkasrawi, Bioconversion of paper mill sludge to bioethanol in the presence of accelerants or hydrogen peroxide pretreatment. Bioresour. Technol. 192, 529–539 (2015). https://doi.org/10.1016/j.biortech.2015.06.010
K. Mase, M. Yoneda, Y. Yamada, S. Fukuzumi, Seawater usable for production and consumption of hydrogen peroxide as a solar fuel. Nat. Commun. 7, 1–7 (2016). https://doi.org/10.1038/ncomms11470
Y.Q. Lan, X.L. Wang, L.Z. Dong, Q. Man, J.X. Su, Exploring the performance improvement of oxygen evolution reaction in stable bimetal–organic framework system. Angew. Chem. Int. Ed. 57, 9660–9664 (2018). https://doi.org/10.1002/anie.201803587
Z. Zhang, K. Liu, Z. Feng, Y. Bao, B. Dong, Hierarchical sheet-on-sheet ZnIn2S4/g-C3N4 heterostructure with highly efficient photocatalytic H2 production based on photoinduced interfacial charge transfer. Sci. Rep. 6, 19221 (2016). https://doi.org/10.1038/srep19221
C.Z. Liu, Y.F. Zhang, X.F. Li, X.F. Lu, Z. Chang et al., The high energy X-ray telescope (HE) onboard the insight-HXMT astronomy satellite. Sci. China Phys. Mech. 63, 249503 (2020). https://doi.org/10.1007/s11433-019-1486-x
M. Zhong, T. Hisatomi, Y. Kuang, J. Zhao, M. Liu et al., Surface modification of CoOx loaded BiVo4 photoanodes with ultrathin p-type NiO layers for improved solar water oxidation. J. Am. Chem. Soc. 137, 5053–5060 (2015). https://doi.org/10.1021/jacs.5b00256
L. Wang, X. Zheng, L. Chen, Y. Xiong, H. Xu, Van der Waals heterostructures comprised of ultrathin polymer nanosheets for efficient z-scheme overall water splitting. Angew. Chem. Int. Ed. 130, 3512–3516 (2018). https://doi.org/10.1002/ange.201710557
M. Zhu, S. Kim, L. Mao, M. Fujitsuka, J. Zhang, X. Wang, T. Majima, Metal-free photocatalyst for H2 evolution in visible to near-infrared region: black phosphorus/graphitic carbon nitride. J. Am. Chem. Soc. 139, 13234–13242 (2017). https://doi.org/10.1021/jacs.5b00256
J. Ran, W. Guo, H. Wang, B. Zhu, J. Yu, S.Z. Qiao, Metal-free 2D/2D phosphorene/g-C3N4 van der Waals heterojunction for highly enhanced visible-light photocatalytic h2 production. Adv. Mater. 30, 1800128 (2018). https://doi.org/10.1002/adma.201800128
Q. Gai, X. Zheng, W. Liu, Q. Dong, Y. Wang, R. Gao, S. Ren, 2D–2D heterostructured CdS–CoP photocatalysts for efficient H2 evolution under visible light irradiation. Int. J. Hydrog. Energy 44, 27412–27420 (2019). https://doi.org/10.1016/j.ijhydene.2019.08.196
R. Sasikala, A. Gaikwad, O. Jayakumar, K. Girija, R. Rao, A. Tyagi, S. Bharadwaj, Nanohybrid MoS2-PANI-CdS photocatalyst for hydrogen evolution from water. Colloids Surf. A 481, 485–492 (2015). https://doi.org/10.1016/j.colsurfa.2015.06.027
M. Luo, W. Yao, C. Huang, Q. Wu, Q. Xu, Shape effects of pt nanoparticles on hydrogen production via Pt/CdS photocatalysts under visible light. J. Mater. Chem A 3, 13884–13891 (2015). https://doi.org/10.1039/C5TA00218D
D. Lang, T. Shen, Q. Xiang, Roles of MoS2 and graphene as cocatalysts in the enhanced visible-light photocatalytic H2 production activity of multiarmed CdS nanorods. ChemCatChem 7, 943–951 (2015). https://doi.org/10.1002/cctc.201403062
W. Jiang, Y. Liu, R. Zong, Z. Li, W. Yao, Y. Zhu, Photocatalytic hydrogen generation on bifunctional ternary heterostructured In2S3/MoS2/CdS composites with high activity and stability under visible light irradiation. J. Mater. Chem. A 3, 18406–18412 (2015). https://doi.org/10.1039/C5TA04258E
T. Jia, A. Kolpin, C. Ma, R.C.T. Chan, W.M. Kwok, S.E. Tsang, A graphene dispersed CdS–MoS2 nanocrystal ensemble for cooperative photocatalytic hydrogen production from water. Chem. Commun. 50, 1185–1188 (2014). https://doi.org/10.1039/C3CC47301E
K. Chang, Z. Mei, T. Wang, Q. Kang, S. Ouyang, J. Ye, MoS2/graphene cocatalyst for efficient photocatalytic H2 evolution under visible light irradiation. ACS Nano 8, 7078–7087 (2014). https://doi.org/10.1021/nn5019945
K. Chang, M. Li, T. Wang, S. Ouyang, P. Li, L. Liu, J. Ye, Drastic layer number dependent activity enhancement in photocatalytic H2 evolution over nMoS2/CdS (n ≥ 1) under visible light. Adv. Energy Mater. 5, 1402279 (2015). https://doi.org/10.1021/nn5019945
X. Zong, G. Wu, H. Yan, G. Ma, J. Shi et al., Photocatalytic H2 evolution on MoS2/CdS catalysts under visible light irradiation. J. Phys. Chem. C 114, 1963–1968 (2010). https://doi.org/10.1021/jp904350e
X. Zong, H. Yan, G. Wu, G. Ma, F. Wen, L. Wang, C. Li, Enhancement of photocatalytic H2 evolution on CdS by loading MoS2 as cocatalyst under visible light irradiation. J. Am. Chem. Soc. 130, 7176–7177 (2008). https://doi.org/10.1021/ja8007825
S. Ma, J. Xie, J. Wen, K. He, X. Li, W. Liu, X. Zhang, Constructing 2D layered hybrid CdS nanosheets/mos2 heterojunctions for enhanced visible-light photocatalytic H2 generation. Appl. Surf. Sci. 391, 580–591 (2017). https://doi.org/10.1016/j.apsusc.2016.07.067
X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin et al., A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 8, 76–80 (2009). https://doi.org/10.1038/nmat2317
L. Jia, D.H. Wang, Y.X. Huang, A.W. Xu, H.Q. Yu, Highly durable n-doped graphene/cds nanocomposites with enhanced photocatalytic hydrogen evolution from water under visible light irradiation. J. Phys. Chem. C 115, 11466–11473 (2011). https://doi.org/10.1021/jp2023617
U. Maitra, U. Gupta, M. De, R. Datta, A. Govindaraj, C. Rao, Highly effective visible-light-induced H2 generation by single-layer 1T-MoS2 and a nanocomposite of few-layer 2H-MoS2 with heavily nitrogenated graphene. Angew. Chem. Int. Ed. 52, 13057–13061 (2013). https://doi.org/10.1002/anie.201306918
U. Gupta, B. Naidu, U. Maitra, A. Singh, S.N. Shirodkar, U.V. Waghmare, C. Rao, Characterization of few-layer 1T-MoSe2 and its superior performance in the visible-light induced hydrogen evolution reaction. APL Mater. 2, 092802 (2014). https://doi.org/10.1063/1.4892976
B. Mahler, V. Hoepfner, K. Liao, G.A. Ozin, Colloidal synthesis of 1T-WS2 and 2H-WS2 nanosheets: applications for photocatalytic hydrogen evolution. J. Am. Chem. Soc. 136, 14121–14127 (2014). https://doi.org/10.1021/ja506261t
J. Chen, X.J. Wu, L. Yin, B. Li, X. Hong et al., One-pot synthesis of CdS nanocrystals hybridized with single-layer transition-metal dichalcogenide nanosheets for efficient photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 54, 1210–1214 (2015). https://doi.org/10.1002/anie.201410172
Z. Lei, W. You, M. Liu, G. Zhou, T. Takata et al., Photocatalytic water reduction under visible light on a novel ZnIn2S4 catalyst synthesized by hydrothermal method. Chem. Commun. 17, 2142–2143 (2003). https://doi.org/10.1039/B306813G
Z. Xu, Y. Li, S. Peng, G. Lu, S. Li, Nacl-assisted low temperature synthesis of layered Zn–In–S photocatalyst with high visible-light activity for hydrogen evolution. RSC Adv. 2, 3458–3466 (2012). https://doi.org/10.1039/C2RA01159J
J. Yu, L. Qi, M. Jaroniec, Hydrogen production by photocatalytic water splitting over Pt/TiO2 nanosheets with exposed (001) facets. J. Phys. Chem. C 114, 13118–13125 (2010). https://doi.org/10.1021/jp104488b
K. Zhang, M. Fujitsuka, Y. Du, T. Majima, 2D/2D heterostructured CdS/WS2 with efficient charge separation improving H2 evolution under visible light irradiation. ACS Appl. Mater. Interfaces. 10, 20458–20466 (2018). https://doi.org/10.1021/acsami.8b04080
R.P. Schwarzenbach, T. Egli, T.B. Hofstetter, U.V. Gunten, B. Wehrli, Global water pollution and human health. Annu. Rev. Environ. Resour. 35, 10 (2010). https://doi.org/10.1146/annurev-environ-100809-125342
Y. Li, K. Li, Y. Yang, L. Li, Y. Xing et al., Ultrathin g-C3N4 nanosheets coupled with AgIO3 as highly efficient heterostructured photocatalysts for enhanced visible-light photocatalytic activity. Chemistry 21, 17739–17747 (2015). https://doi.org/10.1002/chem.201502945
J. Wang, L. Tang, G. Zeng, Y. Deng, Y. Liu et al., Atomic scale g-C3N4/Bi2WO6 2D/2D heterojunction with enhanced photocatalytic degradation of ibuprofen under visible light irradiation. Appl. Catal. B Environ. 209, 285–294 (2017). https://doi.org/10.1016/j.apcatb.2017.03.019
R. Bera, S. Kundu, A. Patra, 2D hybrid nanostructure of reduced graphene oxide–CdS nanosheet for enhanced photocatalysis. ACS Appl. Mater. Interfaces. 7, 13251 (2015). https://doi.org/10.1021/acsami.5b03800
Y. Liu, S. Xie, L. Hui, X. Wang, A highly efficient sunlight driven ZnO nanosheet photocatalyst: synergetic effect of p-doping and MoS2 atomic layer loading. ChemCatChem 6, 2522–2526 (2014). https://doi.org/10.1002/cctc.201402191
W.J. Ong, L.L. Tan, S.P. Chai, S.T. Yong, Graphene oxide as a structure-directing agent for the two-dimensional interface engineering of sandwich-like graphene-g-C3N4 hybrid nanostructures with enhanced visible-light photoreduction of CO2 to methane. Chem. Commun. 51, 858–861 (2015). https://doi.org/10.1039/c4cc08996k
J. Sun, H. Zhang, L.H. Guo, L. Zhao, Two-dimensional interface engineering of a titania–graphene nanosheet composite for improved photocatalytic activity. ACS Appl. Mater. Interfaces. 5, 13035–13041 (2013). https://doi.org/10.1021/am403937y
W. Ong, L.L. Tan, S.P. Chai, S.T. Yong, A. Mohamed, Surface charge modification via protonation of graphitic carbon nitride (g-C3N4) for electrostatic self-assembly construction of 2D/2D reduced graphene oxide (rGO)/g-C3N4 nanostructures toward enhanced photocatalytic reduction of carbon dioxide to methane. Nano Energy 13, 757–770 (2015). https://doi.org/10.1016/j.nanoen.2015.03.014
Y.T. Liang, B.K. Vijayan, O. Lyandres, K.A. Gray, M.C. Hersam, Effect of dimensionality on the photocatalytic behavior of carbon-titania nanosheet composites: charge transfer at nanomaterial interfaces. J. Phys. Chem. Lett. 3, 1760–1765 (2012). https://doi.org/10.1021/jz300491s
W. Tu, Y. Zhou, Q. Liu, Z. Tian, J. Gao et al., Robust hollow spheres consisting of alternating titania nanosheets and graphene nanosheets with high photocatalytic activity for CO2 conversion into renewable fuels. Adv. Funct. Mater. 22, 1215–1221 (2012). https://doi.org/10.1002/adfm.201102566
X. Chen, Y. Zhou, Q. Liu, Z. Li, J. Liu, Z. Zou, Ultrathin, single-crystal WO3 nanosheets by two-dimensional oriented attachment toward enhanced photocatalystic reduction of CO2 into hydrocarbon fuels under visible light. ACS Appl. Mater. Interfaces. 4, 3372–3377 (2012). https://doi.org/10.1021/am300661s
W. Tu, Y. Li, L. Kuai, Y. Zhou, Q. Xu et al., Construction of unique two-dimensional MoS2–TiO2 hybrid nanojunctions: MoS2 as a promising cost-effective cocatalyst toward improved photocatalytic reduction of CO2 to methanol. Nanoscale 9, 9065–9070 (2017). https://doi.org/10.1039/C7NR03238B
L. Zhang, W. Wang, D. Jiang, E. Gao, S. Sun, Photoreduction of CO2 on BioCl nanoplates with the assistance of photoinduced oxygen vacancies. Nano Res. 8, 821–831 (2015). https://doi.org/10.1007/s12274-014-0564-2
J.C. Wang, H.C. Yao, Z.Y. Fan, L. Zhang, J.S. Wang, S.Q. Zang, Z.J. Li, Indirect z-scheme BiOi/g-C3N4 photocatalysts with enhanced photoreduction CO2 activity under visible light irradiation. ACS Appl. Mater. Interfaces. 8, 3765–3775 (2016). https://doi.org/10.1021/acsami.5b09901
S. Kawamura, M.C. Puscasu, Y. Yoshida, Y. Izumi, G. Carja, Tailoring assemblies of plasmonic silver/gold and zinc–gallium layered double hydroxides for photocatalytic conversion of carbon dioxide using UV–visible light. Appl. Catal. A Gen. 504, 238–247 (2015). https://doi.org/10.1016/j.apcata.2014.12.042
H.C. Hsu, I. Shown, H.Y. Wei, Y.C. Chang, H.Y. Du et al., Graphene oxide as a promising photocatalyst for CO2 to methanol conversion. Nanoscale 5, 262–268 (2013). https://doi.org/10.1039/C2NR31718D
B. Qiu, Q. Li, B. Shen, M. Xing, J. Zhang, Stöber-like method to synthesize ultradispersed Fe3O4 nanoparticles on graphene with excellent photo-fenton reaction and high-performance lithium storage. Appl. Catal. B Environ. 183, 216–223 (2016). https://doi.org/10.1016/j.apcatb.2015.10.053
L.L. Tan, W.J. Ong, S.P. Chai, B.T. Goh, A.R. Mohamed, Visible-light-active oxygen-rich TiO2 decorated 2D graphene oxide with enhanced photocatalytic activity toward carbon dioxide reduction. Appl. Catal. B Environ. 179, 160–170 (2015). https://doi.org/10.1016/j.apcatb.2015.05.024
A. Ali, W.C. Oh, A simple ultrasono-synthetic route of PbSe–graphene–TiO2 ternary composites to improve the photocatalytic reduction of CO2. Fuller. Nanotube Carbon Nanostruct. 25, 449–458 (2017). https://doi.org/10.1080/1536383X.2017.1308354
L.L. Tan, W.J. Ong, S.P. Chai, A.R. Mohamed, Reduced graphene oxide–TiO2 nanocomposite as a promising visible-light-active photocatalyst for the conversion of carbon dioxide. Nanoscale Res. Lett. 8, 465 (2013). https://doi.org/10.1186/1556-276X-8-465
A. Wang, X. Li, Y. Zhao, W. Wu, J. Chen, H. Meng, Preparation and characterizations of Cu2O/reduced graphene oxide nanocomposites with high photo-catalytic performances. Powder Technol. 261, 42–48 (2014). https://doi.org/10.1016/j.powtec.2014.04.004
Y.F. Xu, M.Z. Yang, B.X. Chen, X.D. Wang, H.Y. Chen, D.B. Kuang, C.Y. Su, A CsPbBr3 perovskite quantum dot/graphene oxide composite for photocatalytic co2 reduction. J. Am. Chem. Soc. 139, 5660–5663 (2017). https://doi.org/10.1021/jacs.7b00489
L. Kuai, Y. Zhou, W. Tu, P. Li, H. Li et al., Rational construction of a CdS/reduced graphene oxide/TiO2 core-shell nanostructure as an all-solid-state z-scheme system for CO2 photoreduction into solar fuels. RSC Adv. 5, 88409–88413 (2015). https://doi.org/10.1039/C5RA14374H
W. Dai, J. Yu, Y. Deng, X. Hu, T. Wang, X. Luo, Facile synthesis of MoS2/Bi2WO6 nanocomposites for enhanced CO2 photoreduction activity under visible light irradiation. Appl. Surf. Sci. 403, 230–239 (2017). https://doi.org/10.1016/j.apsusc.2017.01.171
X. Jiao, X. Li, X. Jin, Y. Sun, J. Xu et al., Partially oxidized sns2 atomic layers achieving efficient visible-light-driven CO2 reduction. J. Am. Chem. Soc. 139, 18044–18051 (2017). https://doi.org/10.1021/jacs.7b10287
Q. Xin, H. Shah, A. Nawaz, W. Xie, M.Z. Akram et al., Antibacterial carbon-based nanomaterials. Adv. Mater. 31, 1804838 (2019). https://doi.org/10.1002/adma.201804838
J. Ge, Y. Zhang, S.J. Park, Recent advances in carbonaceous photocatalysts with enhanced photocatalytic performances: a mini review. Materials 12, 1916 (2019). https://doi.org/10.3390/ma12121916
R. Wang, X. Zhang, F. Li, D. Cao, M. Pu et al., Energy-level dependent H2O2 production on metal-free, carbon-content tunable carbon nitride photocatalysts. J. Energy Chem. 27, 343–350 (2018). https://doi.org/10.1016/j.jechem.2017.12.014
D. Tasis, N. Tagmatarchis, A. Bianco, M. Prato, Chemistry of carbon nanotubes. Chem. Rev. 106, 1105–1136 (2006). https://doi.org/10.1021/cr050569o
S. Zhao, T. Guo, X. Li, T. Xu, B. Yang, X. Zhao, Carbon nanotubes covalent combined with graphitic carbon nitride for photocatalytic hydrogen peroxide production under visible light. Appl. Catal. B Environ. 224, 725–732 (2018). https://doi.org/10.1016/j.apcatb.2017.11.005
D.L. Long, E. Burkholder, L. Cronin, Polyoxometalate clusters, nanostructures and materials: from self assembly to designer materials and devices. Chem. Soc. Rev. 36, 105–121 (2007). https://doi.org/10.1039/B502666K
X.B. Han, Z.M. Zhang, T. Zhang, Y.G. Li, W. Lin et al., Polyoxometalate-based cobalt–phosphate molecular catalysts for visible light-driven water oxidation. J. Am. Chem. Soc. 136, 5359–5366 (2014). https://doi.org/10.1021/ja412886e
X.J. Kong, Z. Lin, Z.M. Zhang, T. Zhang, W. Lin, Hierarchical integration of photosensitizing metal–organic frameworks and nickel-containing polyoxometalates for efficient visible-light-driven hydrogen evolution. Angew. Chem. Int. Ed. 55, 6411–6416 (2016). https://doi.org/10.1002/anie.201600431
J. Zhou, W. Chen, C. Sun, L. Han, C. Qin et al., Oxidative polyoxometalates modified graphitic carbon nitride for visible-light CO2 reduction. ACS Appl. Mater. Interfaces. 9, 11689–11695 (2017). https://doi.org/10.1021/acsami.7b01721
S. Zhao, X. Zhao, H. Zhang, J. Li, Y. Zhu, Covalent combination of polyoxometalate and graphitic carbon nitride for light-driven hydrogen peroxide production. Nano Energy 35, 405–414 (2017). https://doi.org/10.1016/j.nanoen.2017.04.017
S. Zhao, X. Zhao, S. Ouyang, Y. Zhu, Polyoxometalates covalently combined with graphitic carbon nitride for photocatalytic hydrogen peroxide production. Catal. Sci. Technol. 8, 1686–1695 (2018). https://doi.org/10.1039/C8CY00043C
S. Zhao, X. Zhao, Polyoxometalates-derived metal oxides incorporated into graphitic carbon nitride framework for photocatalytic hydrogen peroxide production under visible light. J. Catal. 366, 98–106 (2018). https://doi.org/10.1016/j.jcat.2018.08.003
S. Zhao, X. Zhao, Insights into the role of singlet oxygen in the photocatalytic hydrogen peroxide production over polyoxometalates-derived metal oxides incorporated into graphitic carbon nitride framework. Appl. Catal. B Environ. 250, 408–418 (2019). https://doi.org/10.1016/j.apcatb.2019.02.031
X. Jiang, P. Wang, J. Zhao, 2d covalent triazine framework: a new class of organic photocatalyst for water splitting. J. Mater. Chem. A 3, 7750–7758 (2015). https://doi.org/10.1039/C4TA03438D
Y. Shiraishi, S. Kanazawa, Y. Kofuji, H. Sakamoto, S. Ichikawa, S. Tanaka, T. Hirai, Sunlight-driven hydrogen peroxide production from water and molecular oxygen by metal-free photocatalysts. Angew. Chem. Int. Ed. 53, 13454–13459 (2014). https://doi.org/10.1002/anie.201407938
Y. Kofuji, S. Ohkita, Y. Shiraishi, H. Sakamoto, S. Tanaka, S. Ichikawa, T. Hirai, Graphitic carbon nitride doped with biphenyl diimide: efficient photocatalyst for hydrogen peroxide production from water and molecular oxygen by sunlight. ACS Catal. 6, 7021–7029 (2016). https://doi.org/10.1021/acscatal.6b02367
Y. Kofuji, S. Ohkita, Y. Shiraishi, H. Sakamoto, S. Ichikawa, S. Tanaka, T. Hirai, Mellitic triimide-doped carbon nitride as sunlight-driven photocatalysts for hydrogen peroxide production. Catal. Sci. Technol. 5, 6478–6485 (2017). https://doi.org/10.1021/acssuschemeng.7b00575
Y. Kofuji, Y. Isobe, Y. Shiraishi, H. Sakamoto, S. Tanaka, S. Ichikawa, T. Hirai, Carbon nitride–aromatic diimide–graphene nanohybrids: metal-free photocatalysts for solar-to-hydrogen peroxide energy conversion with 0.2% efficiency. J. Am. Chem. Soc. 138, 10019–10025 (2016). https://doi.org/10.1021/jacs.6b05806
Y. Kofuji, Y. Isobe, Y. Shiraishi, H. Sakamoto, S. Ichikawa, S. Tanaka, T. Hirai, Hydrogen peroxide production on a carbon nitride–boron nitride-reduced graphene oxide hybrid photocatalyst under visible light. ChemCatChem 10, 2070–2077 (2018). https://doi.org/10.1002/cctc.201701683
L. Yang, G. Dong, D.L. Jacobs, Y. Wang, L. Zang, C. Wang, Two-channel photocatalytic production of H2O2 over g-C3N4 nanosheets modified with perylene imides. J. Catal. 352, 274–281 (2017). https://doi.org/10.1016/j.jcat.2017.05.010
H.I. Kim, Y. Choi, S. Hu, W. Choi, J.H. Kim, Photocatalytic hydrogen peroxide production by anthraquinone-augmented polymeric carbon nitride. Appl. Catal. B Environ. 229, 121–129 (2018). https://doi.org/10.1016/j.apcatb.2018.01.060
F. Xue, Y. Si, M. Wang, M. Liu, L. Guo, Toward efficient photocatalytic pure water splitting for simultaneous H2 and H2O2 production. Nano Energy 62, 823–831 (2019). https://doi.org/10.1016/j.nanoen.2019.05.086
Y. Shiraishi, S. Kanazawa, Y. Sugano, D. Tsukamoto, H. Sakamoto, S. Ichikawa, T. Hirai, Highly selective production of hydrogen peroxide on graphitic carbon nitride (g-C3N4) photocatalyst activated by visible light. ACS Catal. 4, 774–780 (2014). https://doi.org/10.1021/cs401208c
B.O. Burek, D.W. Bahnemann, J.Z. Bloh, Modeling and optimization of the photocatalytic reduction of molecular oxygen to hydrogen peroxide over titanium dioxide. ACS Catal. 9, 25–37 (2018). https://doi.org/10.1021/acscatal.8b03638
R. Cai, Y. Kubota, A. Fujishima, Effect of copper ions on the formation of hydrogen peroxide from photocatalytic titanium dioxide particles. J. Catal. 219, 214–218 (2003). https://doi.org/10.1016/S0021-9517(03)00197-0
S. Kim, G.H. Moon, G. Kim, U. Kang, H. Park, W. Choi, TiO2 complexed with dopamine-derived polymers and the visible light photocatalytic activities for water pollutants. J. Catal. 346, 92–100 (2017). https://doi.org/10.1016/j.jcat.2016.11.027
X. Zeng, Z. Wang, G. Wang, T.R. Gengenbach, D.T. McCarthy et al., Highly dispersed TiO2 nanocrystals and wo3 nanorods on reduced graphene oxide: Z-scheme photocatalysis system for accelerated photocatalytic water disinfection. Appl. Catal. B Environ. 218, 163–173 (2017). https://doi.org/10.1016/j.apcatb.2017.06.055
X. Xiong, X. Zhang, S. Liu, J. Zhao, Y. Xu, Sustained production of H2O2 in alkaline water solution using borate and phosphate-modified Au/TiO2 photocatalysts. Photochem. Photobiol. Sci. 17, 1018–1022 (2018). https://doi.org/10.1039/C8PP00177D
G. Zuo, B. Li, Z. Guo, L. Wang, F. Yang et al., Efficient photocatalytic hydrogen peroxide production over TiO2 passivated by SnO2. Catalysts 9, 623 (2019). https://doi.org/10.3390/catal9070623
Y. Shiraishi, Y. Kofuji, H. Sakamoto, S. Tanaka, S. Ichikawa, T. Hirai, Effects of surface defects on photocatalytic H2O2 production by mesoporous graphitic carbon nitride under visible light irradiation. ACS Catal. 5, 3058–3066 (2015). https://doi.org/10.1021/acscatal.5b00408
S. Kim, G.H. Moon, H. Kim, Y. Mun, P. Zhang, J. Lee, W. Choi, Selective charge transfer to dioxygen on KPF6-modified carbon nitride for photocatalytic synthesis of H2O2 under visible light. J. Catal. 357, 51–58 (2018). https://doi.org/10.1016/j.jcat.2017.10.002
L. Shi, L. Yang, W. Zhou, Y. Liu, L. Yin et al., Photoassisted construction of holey defective g-C3N4 photocatalysts for efficient visible-light-driven H2O2 production. Small 14, 1703142 (2018). https://doi.org/10.1002/smll.201703142
Z. Wei, M. Liu, Z. Zhang, W. Yao, H. Tan, Y. Zhu, Efficient visible-light-driven selective oxygen reduction to hydrogen peroxide by oxygen-enriched graphitic carbon nitride polymers. Energy Environ. Sci. 11, 2581–2589 (2018). https://doi.org/10.1039/C8EE01316K
Y. Kusuyama, S. Tanaka, K. Sakatsuji, T. Nishihara, K. Saito et al., Central pontine myelinolysis. Pathol. Int. 32, 725–732 (2008). https://doi.org/10.1111/j.1440-1827.1982.tb02074.x
S. Zhao, X. Wang, M. Huo, Catalytic wet air oxidation of phenol with air and micellar molybdovanadophosphoric polyoxometalates under room condition. Appl. Catal. B Environ. 97, 127–134 (2010). https://doi.org/10.1016/j.apcatb.2010.03.032
S. Kato, J. Jung, T. Suenobu, S. Fukuzumi, Production of hydrogen peroxide as a sustainable solar fuel from water and dioxygen. Energy Environ. Sci. 6, 3756–3764 (2013). https://doi.org/10.1039/C3EE42815J
H. Hirakawa, S. Shiota, Y. Shiraishi, H. Sakamoto, S. Ichikawa, T. Hirai, Au nanoparticles supported on BiVO4: effective inorganic photocatalysts for H2O2 production from water and O2 under visible light. ACS Catal. 6, 4976–4982 (2016). https://doi.org/10.1021/acscatal.6b01187
X. Jie, Z. Chen, H. Zhang, G. Lin, H. Lin, X. Wang, J. Long, Cd3(C3N3S3)2 coordination polymer/graphene nanoarchitectures for enhanced photocatalytic H2O2 production under visible light. Sci. Bull. 62, 610–618 (2017). https://doi.org/10.1016/j.scib.2017.04.013
T. Inagami, N. Yokosawa, N. Takahashi, Y. Takii, Partial purification of prorenin and activation by kallikreins: a possible new link between renin and kallikrein systems. Adv. Exp. Med. Biol. 120, 415–428 (1979)
Acknowledgements
The research was partially supported by the National Natural Science Fund of China (Grant Nos. 61875138, 61435010, and 61961136001).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khan, K., Tareen, A.K., Aslam, M. et al. Recent Progress, Challenges, and Prospects in Two-Dimensional Photo-Catalyst Materials and Environmental Remediation. Nano-Micro Lett. 12, 167 (2020). https://doi.org/10.1007/s40820-020-00504-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40820-020-00504-3