Abstract
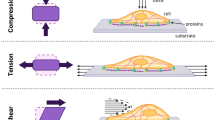
Serious wounds, both chronic and acute (e.g., surgical), are among the most common, expensive and difficult-to-treat health problems. Negative pressure wound therapy (NPWT) is considered a mainstream procedure for treating both wound types. Soft tissue deformation stimuli are the crux of NPWT, enhancing cell proliferation and migration from peri-wound tissues which contributes to healing. We developed a dynamic stretching device (DSD) contained in a miniature incubator for applying controlled deformations to fibroblast wound assays. Prior to the stretching experiments, fibroblasts were seeded in 6-well culture plates with elastic substrata and let to reach confluency. Squashing damage was then induced at the culture centers, and the DSD was activated to deliver stretching regimes that represented common clinical NPWT protocols at two peak strain levels, 0.5% and 3%. Analyses of the normalized maximal migration rate (MMR) data for the collective cell movement revealed that for the 3% strain level, the normalized MMR of cultures subjected to a 0.1 Hz stretch frequency regime was ~ 1.4 times and statistically significantly greater (p < 0.05) than that of the cultures subjected to no-stretch (control) or to static stretch (2nd control). Correspondingly, analysis of the time to gap closure data indicated that the closure time of the wound assays subjected to the 0.1 Hz regime was ~ 30% shorter than that of the cultures subjected to the control regimes (p < 0.05). Other simulated NPWT protocols did not emerge as superior to the controls. The present method and system are a powerful platform for further revealing the mechanobiology of NPWT and for improving this technology.





Similar content being viewed by others
Code availability
The Matlab code used for the present analyses is available.
Data availability and materials
The data and materials are available.
References
Angelini TE, Hannezo E, Trepat X, Fredberg JJ, Weitz DA (2010) Cell migration driven by cooperative substrate deformation patterns. Phys Rev Lett 104:168104
Ao M, Brewer BM, Yang L, Franco Coronel OE, Hayward SW, Webb DJ, Li D (2015) Stretching fibroblasts remodels fibronectin and alters cancer cell migration. Sci Rep 9(5):8334
Apelqvist J, Willy C, Fagerdahl AM et al (2017) Negative pressure wound therapy-overview, challenges and perspectives. J Wound Care 26(3 Suppl 3):S1–S113
Be'ery-Lipperman M, Gefen A (2005) Contribution of muscular weakness to osteoporosis: computational and animal models. Clin Biomech 20(9):984–997
Chanet S, Martin AC (2014) Mechanical force sensing in tissues. Prog Mol Biol Transl Sci 126:317–352
Chow SE, Chen CP, Hsu CC, Tsai WC, Wang JS, Hsu NC (2016) Quantifying cell behaviors in negative-pressure induced monolayer cell movement. Biomed J 39(1):50–59
Dastouri P, Helm DL, Scherer SS, Pietramaggiori G, Younan G, Orgill DP (2011) Waveform modulation of negative-pressure wound therapy in the murine model. Plast Reconstr Surg 127(4):1460–1466
Desai LP, White SR, Waters CM (2010) Cyclic mechanical stretch decreases cell migration by inhibiting phosphatidylinositol 3-kinase- and focal adhesion kinasemediated JNK1 activation. J Biol Chem 285:4511–4519
Fronza M, Heinzmann B, Hamburger M, Laufer S, Merfort I (2009) Determination of the wound healing effect of Calendula extracts using the scratch assay with 3T3 fibroblasts. J Ethnopharmacol 126(3):463–467
Geddes DM, Cargill RS 2nd (2001) An in vitro model of neural trauma: device characterization and calcium response to mechanical stretch. J Biomech Eng 123(3):247–255
Gefen A, Alves P, Ciprandi G et al (2020) Device-related pressure ulcers: SECURE prevention. J Wound Care 29(Sup2a):S1–S52
Gefen A, Brienza D, Edsberg L, Milton W, Murphy C, Oomens CWJ, Perry L, Sari Y (2019a) The etiology of pressure injuries. In: Prevention and treatment of pressure ulcers/injuries: clinical practice guideline European Pressure Ulcer Advisory Panel (EPUAP), National Pressure Injury Advisory Panel (NPIAP) and the Pan Pacific Pressure Injury Alliance (PPPIA), 3rd Edition
Gefen A (2019b) How medical engineering has changed our understanding of chronic wounds and future prospects. Med Eng Phys 72:13–18
Ghazanfari S, Tafazzoli-Shadpour M, Shokrgozar MA (2009) Effects of cyclic stretch on proliferation of mesenchymal stem cells and their differentiation to smooth muscle cells. Biochem Biophys Res Commun 388(3):601–605
Hirano Y, Ishiguro N, Sokabe M, Takigawa M, Naruse K (2008) Effects of tensile and compressive strains on response of a chondrocytic cell line embedded in type I collagen gel. J Biotechnol 133:245–252
Hong JP (2013) Addressing the vertical and horizontal aspects of the wound by using negative pressure wound therapy and growth factors. Wounds Int 4(4):6–7
Huang C, Leavitt T, Bayer LR, Orgill DP (2014) Effect of negative pressure wound therapy on wound healing. Curr Probl Surg 51:301–331
Kato T, Ishiguro N, Iwata H, Kojima T, Ito T, Naruse K (1998) Up-regulation of COX2 expression by uni-axial cyclic stretch in human lung fibroblast cells. Biochem Biophys Res Commun 244:615–619
Katzengold R, Shoham N, Benayahu D, Gefen A (2015) Simulating single cell experiments in mechanical testing of adipocytes. Biomech Model Mechanobiol 14(3):537–547
Katzengold R, Topaz M, Gefen A (2018) Dynamic computational simulations for evaluating tissue loads applied by regulated negative pressure-assisted wound therapy (RNPT) system for treating large wounds. J Tissue Viability 27(2):101–113
Lalezari S, Lee CJ, Borovikova AA, Banyard DA, Paydar KZ, Wirth GA, Widgerow AD (2017) Deconstructing negative pressure wound therapy. Int Wound J 14(4):649–657
Lee AA, Delhaas T, Waldman LK, MacKenna DA, Villarreal FJ, McCulloch AD (1996) An equibiaxial strain system for cultured cells. Am J Physiol 271(4 Pt 1):C1400–C1408
Lee KN, Ben-Nakhi M, Park EJ, Hong JP (2015) Cyclic negative pressure wound therapy: an alternative mode to intermittent system. Int Wound J 12(6):686–692
Leopold E, Gefen A (2013) Changes in permeability of the plasma membrane of myoblasts to fluorescent dyes with different molecular masses under sustained uniaxial stretching. Med Eng Phys 35(5):601–607
Levy A, Enzer S, Shoham N, Zaretsky U, Gefen A (2012) Large, but not small sustained tensile strains stimulate adipogenesis in culture. Ann Biomed Eng 40(5):1052–1060
Liang CC, Park AY, Guan JL (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2(2):329–333
Lin JY, Lo KY, Sun YS (2019) Effects of substrate-coating materials on the wound-healing process. Materials 12(17):2775
Marom A, Berkovitch Y, Toume S, Alvarez-Elizondo MB, Weihs D (2019) Non-damaging stretching combined with sodium pyruvate supplement accelerate migration of fibroblasts and myoblasts during gap closure. Clin Biomech 62:96–103
Monsuur HN, Boink MA, Weijers EM, Roffel S, Breetveld M, Gefen A, van den Broek LJ, Gibbs S (2016) Methods to study differences in cell mobility during skin wound healing in vitro. J Biomech 49(8):1381–1387
Nagai Y, Yokoi H, Kaihara K, Naruse K (2012) The mechanical stimulation of cells in 3D culture within a self-assembling peptide hydrogel. Biomaterials 33:1044–1051
Naruse K, Yamada T, Sokabe M (1998) Involvement of SA channels in orienting response of cultured endothelial cells to cyclic stretch. Am J Phys 274(5 pt 2):H1532–H1538
Nussbaum SR, Carter MJ, Fife CE, DaVanzo J, Haught R, Nusgart M, Cartwright D (2018) An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health 21(1):27–32
Osada T, Watanabe S, Tanaka H, Hirose M, Miyazaki A, Sato N (1999) Effect of mechanical strain on gastric cellular migration and proliferation during mucosal healing: role of Rho dependent and Rac dependent cytoskeletal reorganisation. Gut 45:508–515
Othman D (2012) Negative pressure wound therapy literature review of efficacy, cost effectiveness, and impact on patients' quality of life in chronic wound management and its implementation in the United kingdom. Plast Surg Int 2012:374398
Padula WV, Delarmente BA (2019) The national cost of hospital-acquired pressure injuries in the United States. Int Wound J 16(3):634–640
Paluch EK, Nelson CM, Biais N et al (2015) Mechanotransduction: use the force(s). BMC Biol 13:47
Pandit V, Nesbitt SR, Kim DY, Mixon A, Kotha SP (2015) Combinatorial therapy using negative pressure and varying lithium dosage for accelerated wound healing. J Mech Behav Biomed Mater 44:173–178
Pijuan J, Barceló C, Moreno DF, Maiques O, Sisó P, Marti RM, Macià A, Panosa A (2019) In vitro cell migration, invasion, and adhesion assays: from cell imaging to data analysis. Front Cell Dev Biol 7:107
Pinto BI, Cruz ND, Lujan OR, Propper CR, Kellar RS (2019) In vitro scratch assay to demonstrate effects of arsenic on skin cell migration. J Vis Exp 144:e58838
Rajagopalan P, Marganski WA, Brown XQ, Wong JY (2004) Direct comparison of the spread area, contractility, and migration of balb/c 3T3 fibroblasts adhered to fibronectin- and RGD-modified substrata. Biophys J 87(4):2818–2827
Rodriguez-Menocal L, Salgado M, Ford D, Van Badiavas E (2012) Stimulation of skin and wound fibroblast migration by mesenchymal stem cells derived from normal donors and chronic wound patients. Stem Cells Transl Med 1(3):221–229
Shkolyar A, Gefen A, Benayahu D, Greenspan H (2015) Automatic detection of cell divisions (mitosis) in live-imaging microscopy images using convolutional neural networks. Conf Proc IEEE Eng Med Biol Soc 2015:743–746
Shoham N, Gottlieb R, Sharabani-Yosef O, Zaretsky U, Benayahu D, Gefen A (2012) Static mechanical stretching accelerates lipid production in 3T3-L1 adipocytes by activating the MEK signaling pathway. Am J Physiol Cell Physiol 302(2):C429–C441
Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341(10):738–746
Slomka N, Gefen A (2012) Relationship between strain levels and permeability of the plasma membrane in statically stretched myoblasts. Ann Biomed Eng 40:606–618
Stroncek JD, Reichert WM (2008) Overview of wound healing in different tissue types. In: Reichert WM (ed) Indwelling neural implants: strategies for contending with the in vivo environment. CRC Press, Boca Raton
Tai G, Tai M, Zhao M (2018) Electrically stimulated cell migration and its contribution to wound healing. Burns Trauma 6:20
Tokuyama E, Nagai Y, Takahashi K, Kimata Y, Naruse K (2015) Mechanical stretch on human skin equivalents increases the epidermal thickness and develops the basement membrane. PLoS ONE 10:e0141989
Topman G, Sharabani-Yosef O, Gefen A (2011) A method for quick, low-cost automated confluency measurements. Microsc Microanal 17(6):915–922
Topman G, Sharabani-Yosef O, Gefen A (2012a) A standardized objective method for continuously measuring the kinematics of cultures covering a mechanically damaged site. Med Eng Phys 34(2):225–232
Topman G, Lin FH, Gefen A (2012b) The influence of ischemic factors on the migration rates of cell types involved in cutaneous and subcutaneous pressure ulcers. Ann Biomed Eng 40(9):1929–1939
Topman G, Lin FH, Gefen A (2013a) The natural medications for wound healing-curcumin, aloe-vera and ginger—do not induce a significant effect on the migration kinematics of cultured fibroblasts. J Biomech 46(1):170–174
Topman G, Shoham N, Sharabani-Yosef O, Lin FH, Gefen A (2013b) A new technique for studying directional cell migration in a hydrogel-based three-dimensional matrix for tissue engineering model systems. Micron 51:9–12
Toume S, Gefen A, Weihs D (2016) Printable low-cost, sustained and dynamic cell stretching apparatus. J Biomech 49(8):1336–1339
Toume S, Gefen A, Weihs D (2017) Low-level stretching accelerates cell migration into a gap. Int Wound J 14(4):698–703
Trepat X, Chen Z, Jacobson K (2012) Cell migration. Compr Phys 2(4):2369–2392
Undyala VV, DemboM CK, Perrin BJ, Huttenlocher A, Elce JS, Greer PA, Wang YL, Beningo KA (2008) The calpain small subunit regulates cell-substrate mechanical interactions during fibroblast migration. J Cell Sci 121(pt 21):3581–3588
van Helvert S, Storm C, Friedl P (2018) Mechanoreciprocity in cell migration. Nat Cell Biol 20(1):8–20
Vikatmaa P, Juutilainen V, Kuukasjärvi P, Malmivaara A (2008) Negative pressure wound therapy: a systematic review on effectiveness and safety. Eur J Vasc Endovasc Surg 36:438–448
Walter MN, Wright KT, Fuller HR, MacNeil S, Johnson WE (2010) Mesenchymal stem cell-conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res 316(7):1271–1281
Wiegand C, White R (2013) Microdeformation in wound healing. Wound Repair Regen 21(6):793–799
Yadav S, Rawal G, Baxi M (2017) Vacuum assisted closure technique: a short review. Pan Afr Med J 28:246
Acknowledgements
We would like to thank Mr. Jelle van Orsouw from Eindhoven University of Technology and Dr. Uri Zaretsky from our Department of Biomedical Engineering at Tel Aviv University for their help regarding the development of the cell stretching apparatus and experimental work. This research work was partially supported by the Israel Science Foundation Grant No. 1266/16 and by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant Agreement No. 811965.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Consent to participate
Not applicable.
Consent for publication
Provided by all authors.
Ethics approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Katzengold, R., Orlov, A. & Gefen, A. A novel system for dynamic stretching of cell cultures reveals the mechanobiology for delivering better negative pressure wound therapy. Biomech Model Mechanobiol 20, 193–204 (2021). https://doi.org/10.1007/s10237-020-01377-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-020-01377-6