Abstract
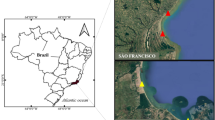
The understanding of the processes that regulates the distribution of the resident macroinfauna on sandy beaches is an urgent need at the current global change scenario. Here, we examine whether the spatial-temporal distribution of the burrowing shrimp Callichirus major (Callichiridae) is affected by the climatic and tidal regimes in two sandy beaches of Brazil (José de Menino and Itaguaré), where is totally prohibited the capture of this species. To this end, we counted shrimp burrows across transects plotted perpendicular to the water line. We found that C. major had a non-random pattern of distribution with dense aggregations of burrows near to the low-tide line. Burrow density and distribution of C. major did not differ across the annual cycle, not following the remarkably environmental seasonality of the southeastern region of Brazil. However, both population parameters were adversely affected when C. major was subject to a high tidal variability. Also, the probability of C. major builds a gallery in intertidal habitats is greater on beaches with very fine sand and low variability of tide amplitude than on beaches with coarser sediments and greater tidal dynamics. Our results point to survival of C. major is not seriously compromised by temperature and precipitation variations, but rather by eventual changes in the tidal regime and sediments. Considering the current scenario of climate change, we argue in favor of C. major may be a good biological model to assess the potential impact of tidal flooding connected to the sea level rise and erosion on coastal habitats.




Similar content being viewed by others
References
Alfredini P, Arasaki E, Do Amaral RF (2008) Mean sea-level rise impacts on Santos Bay, southeastern Brazil–physical modelling study. Environ Monit Assess 144(1-3):377–387. https://doi.org/10.1007/s10661-007-0001-z
Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G (2013) Köppen’s climate classification map for Brazil. Meteorol Z 22(6):711–728. https://doi.org/10.1127/0941-2948/2013/0507
Amaral ACZ, Corte GN, Denadai MR, Colling LA, Borzone C, Veloso V, Omena EP, Zalmon IR, Rocha-Barreira CA, Souza JRB, Rosa LCD, Almeida TCM (2016) Brazilian sandy beaches: characteristics, ecosystem services, impacts, knowledge and priorities. Braz J Oceanogr 64(spe. 2):5–16. https://doi.org/10.1590/S1679-875920160933064sp2
Bertics VJ, Sohm JA, Treude T, Chow CET, Capone DG, Fuhrman JA, Ziebis W (2010) Burrowing deeper into benthic nitrogen cycling: The impact of bioturbation on nitrogen fixation coupled to sulfate reduction. Mar Ecol Prog Ser 409:1–15. https://doi.org/10.3354/meps08639
Botter-Carvalho ML, Santos PJP, Carvalho PVVC (2002) Spatial distribution of Callichirus major (say 1818) (Decapoda, Callianassidae) on a sandy beach, Piedade, Pernambuco, Brazil. Nauplius 10(2):97–109
Celentano E, Defeo O (2006) Habitat harshness and morphodynamics: life history traits of the mole crab Emerita brasiliensis in Uruguayan sandy beaches. Mar Biol 149(6):1453–1461. https://doi.org/10.1007/s00227-006-0309-1
Contreras H, Jaramillo E, Duarte C, McLachlan A (2003) Population abundances, growth and natural mortality of the crustacean macroinfauna at two sand beach morphodynamic types in southern Chile. Rev Chil Hist Nat 76:543–561
Defeo O, McLachlan A (2005) Patterns, processes and regulatory mechanisms in sandy beach macrofauna: a multi-scale analysis. Mar Ecol Prog Ser 295:1–20. https://doi.org/10.3354/meps295001
Defeo O, Rueda R (2002) Spatial structure, sampling design and abundance estimates in sandy beach macroinfauna: some warnings and new perspectives. Mar Biol 140:1215–1225. https://doi.org/10.1007/s00227-002-0783-z
Defeo O, Layerle C, Masello A (1986) Spatial and temporal structure of the ‘yellow clam’ Mesodesma mactroides (Deshayes, 1854) in Uruguay. Med Amb (Chile) 8:48–57
Defeo O, Brazeiro A, de Alava A, Riestra G (1997) Is sandy beach macroinfauna only physically controlled? Role of substrate and competition in isopods. Estuar Coast Shelf Sci 45:453–462. https://doi.org/10.1006/ecss.1996.0200
Dugan JE, Hubbard DM, Rodil IF, Revell DL, Schroeter S (2008) Ecological effects of coastal armoring on sandy beaches. Mar Ecol 29(1):160–170. https://doi.org/10.1111/j.1439-0485.2008.00231.x
Elliott J M (1983) Some Methods for the statistical analysis of samples of benthic invertebrates. Freshwater biological association, scientific publication no 25. 3rd edition. Freshwater biological association, far Sawrey
Felder DL (2001) Diversity and ecological significance of deep-burrowing macrocrustaceans in coastal tropical waters of Americas (Decapoda: Thalassinidea). Interciencia 26:440–449
Frankenberg D, Coles SL, Johannes RE (1967) The potential trophic significance of Callianassa major fecal pellets. Limnol Oceanogr 12(1):113–120. https://doi.org/10.4319/lo.1967.12.1.0113
Gagnon JM, Beaudin L, Silverberg N, Mauviel A (2013) Mesocosm and in situ observations of the burrowing shrimp Calocaris templemani (Decapoda: Thalassinidea) and its bioturbation activities in soft sediments of the Laurentian trough. Mar Biol 160(10):2687–2697. https://doi.org/10.1007/s00227-013-2262-0
Gonçalves SC, Marques JC (2017) Assessment and management of environmental quality conditions in marine sandy beaches for its sustainable use – virtues of the population based approach. Ecol Indic 74:140–146. https://doi.org/10.1016/j.ecolind.2016.11.024
Griffis RB, Suchanek TH (1991) A model of burrow architecture and trophic modes in thalassinidean shrimp (Decapoda: Thalassinidea). Mar Ecol Prog Ser 79:171–183
Guedes CCF, Mendes VR, Giannini PCF, Sawakuchi AO, Nogueira L, Buchmann FS (2017) Idades de deposição dos sedimentos da falésia de Itaguaré (Bertioga-SP) determinadas por luminescência opticamente estimulada. Quat Envi Geosci 8(2):09–16. https://doi.org/10.5380/abequa.v8i2.52474
Hernáez P (2018a) An update on reproduction in ghost shrimps (Decapoda: Axiidea) and mud lobsters (Decapoda: Gebiidea). In: Türkoğlu M, Önal U, Ismen A (eds), Marine Ecology, IntechOpen, pp. 231–253
Hernáez P (2018b). Diversidade e distribuição geográfica de camarões corruptos (Infraordens Axiidea e Gebiidea), ao longo do litoral brasileiro: uma aproximação ecológica aos padrões biogeográficos de distribuição. Cap. 3. Relatório Final, Projeto de Pós-Doutorado FAPESP proc. No 2015/09020–0, pp. 93–116
Hernáez P, Hereman MJ, Pimenta CE, Rio JP, João MC, Pinheiro MA (2019) La efectividad de una ley de protección al servicio de la conservación de un recurso marino: El ejemplo del camarón fantasma Callichirus major (Decapoda, Callianassidae) de la costa de Brasil. Iheringia Ser Zool 109:1–9. https://doi.org/10.1590/1678-4766e2019001
Jaramillo E, González M (1991) Community structure and zonation of the macroinfauna along a dissipative-reflective range of beach category in southern Chile. Stud Neotrop Fauna E 26:193–212. https://doi.org/10.1080/01650529109360853
McLachlan A, Defeo O (2013) Coastal beach ecosystems. In: Levin S (ed) Encyclopedia of biodiversity, 2nd edn. Elsevier Academic Press, Amsterdam, pp 128–136. https://doi.org/10.1016/B978-0-12-384720-1.00294-3
McLachlan A, Jaramillo E, Donn E, Wessels F (1993) Sandy beach macrofauna communities and their control by the physical environment: a geographical comparison. J Coast Res 15:27–38
Melo GAS (1999) Manual de identificação dos Crustacea Decapoda do litoral Brasileiro: Anomura, Thalassinidea, Palinuridea, Astacidea. Plêiade/FAPESP, São Paulo
Moschetto FA, Borges RP, Duarte LFDA (2020) Population structure of Callichirus major (say 1818) (Crustacea: Callianassidae) and conservation considerations at southeast coast of São Paulo, Brazil. An Acad bras Ciênc 92(1). https://doi.org/10.1590/0001-3765202020180795
Najjar RG, Walker HA, Anderson PJ, Barron EJ, Bord RJ, Gibson JR, Kennedy VS, Knight CG, Megonigal JP, O’Connor RE, Polsky CD, Psuty NP, Richards BA, Sorenson LG, Steele EM, Swanson RS (2000) The potential impacts of climate change on the mid-Atlantic coastal region. Clim Res 14(3):219–233. https://doi.org/10.3354/cr014219
Pillay D, Branch G M (2011) Bioengineering effects of burrowing thalassinidean shrimps on marine soft-bottom ecosystems. In: Gibson RN, Atkinson RJA, Gordon JDM (eds), Oceanography and marine biology: an annual review. Volume 49, Taylor & Francis, pp. 137-192
Powilleit M, Graf G (1996) The contribution of the mud shrimp Callianassa subterranea (Decapoda: Thallassinidea) to sediment metabolism during oxygen deficiency in southern North Sea sediments. J Sea Res 36(3-4):193–202. https://doi.org/10.1016/S1385-1101(96)90789-3
Rodil IF, Lastra M (2004) Environmental factors affecting benthic macrofauna along a gradient of intermediate sandy beaches in northern Spain. Estuar Coast Shelf Sci 61:37–44. https://doi.org/10.1016/j.ecss.2004.03.016
Rodrigues SA, Shimizu RM (1997) Autoecology of Callichirus major (say, 1818). Oecol Aust 3(1):155–170
Schlacher TA, Dugan J, Schoeman DS, Lastra M, Jones A, Scapini F, McLachlan A, Defeo O (2007) Sandy beaches at the brink. Divers Distrib 13:556–560. https://doi.org/10.1111/j.1472-4642.2007.00363.x
Short AD (1996) The role of wave height, period, slope, tide range and embaymentisation in beach classifications: a review. Rev Chil Hist Nat 69:589–604
Slott JM, Murray AB, Ashton AD, Crowley TJ (2006) Coastline responses to changing storm patterns. Geophys Res Lett 33(18). https://doi.org/10.1029/2006GL027445
Sokal RR, Rohlf FJ (1981) Biometry. The principles and practice of statistics biological research, 2nd edn. Freeman and Company, San Francisco
Sokal RR, Rohlf FJ (1995) Biometry. The principles and practice of statistics biological research, 3nd edn. Freeman and Company, New York
Souza CRG (2012) Praias arenosas oceânicas do estado de São Paulo (Brasil): síntese dos conhecimentos sobre morfodinâmica, sedimentologia, transporte costeiro e erosão costeira. Rev Depto Geograf 1:308–371. https://doi.org/10.7154/RDG.2012.0112.0014
Souza, JRB, Borzone CA (1996) Distribuição de Calianassídeos (Crustacea: Decapoda: Thalassinidea) em praias do litoral paranaense, com especialreferência a Callichirus major (Say, 1818). Arq Biol Tecnol 39(3):553–565. https://doi.org/10.1590/S0101-81752003000400011
Souza JRB, Borzone CA (2003) A extração de corrupto, Callichirus major (Say) (Crustacea, Thalassinidea), para uso como isca em praias do litoral do Paraná: as populações exploradas. Rev Bras Zool 20(4):625–630. https://doi.org/10.1590/S0101-81752003000400011
Souza JRB, Borzone CA, Brey T (1998) Population dynamics and secondary production of Callichirus major (Crustacea: Thalassinidea) on a southern Brazilian sandy beach. Arch Fish Mar Res 46(2):151–164
Suguio K (1973) Introdução à sedimentologia. EDUSP, São Paulo
Turra A, Croquer A, Carranza A, Mansilla A, Areces AJ, Werlinger C, Martínez-Bayón C, Nassar CAG, Plastino E, Schwindt E, Scarabino F, Chow F, Figueroa FL, Berchez F, Hall-Spencer JM, Soto LA, Buckeridge MS, Copertino M, Széchy MTM, Ghilardi-Lopes NP, Horta P, Coutinho R, Fraschetti S, Nery-Leão ZMA (2013) Global environmental changes: setting priorities for Latin American coastal habitats. Glob Chang Biol 19(7):1965–1969. https://doi.org/10.1111/gcb.12186
Underwood AJ (1997) Experiments in ecology. Cambridge University Press, Cambridge
Ziebis W, Forster S, Huettel M, Jørgensen B (1996) Complex burrows of the mud shrimp Callianassa truncata and their geochemical impact in the sea bed. Nature 382:619–622
Acknowledgements
We wish to thank the FAPESP for the financial support (Process n° 2011/09627-0) and to thank all students and friends who helped during the fieldwork. We are also grateful to Dr. Gray Williams (University of Hong Kong) and Me. Erick Antal Cruz (CECO) for the suggestions in the earlier versions of this paper.
Availability of Data and Material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This study was funded by FAPESP - Fundação de Amparo à Pesquisa do Estado de São Paulo (Process n° 2011/09627–0).
Author information
Authors and Affiliations
Contributions
Ivan R. A. Laurino contributes with: Conceptualization, Methodology, Formal analysis and investigation, Writing - original draft preparation, Funding acquisition. Francisco S. Buchmann contributes with: Conceptualization, Methodology, Investigation, Writing - review and editing, Funding acquisition, Resources, Supervision. Patricio Hernáez contributes with: Formal analysis and investigation, Writing - original draft preparation, Writing - review and editing, Supervision.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
Not applicable because no animal was sampled during the present work.
Consent to Participate
The authors declare that they agreed to participate of the present article.
Consent for Publication
The authors declare that they agreed to the publication of the present article.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 240 kb)
Rights and permissions
About this article
Cite this article
Laurino, I.R.A., Buchmann, F.S. & Hernáez, P. Spatial-Temporal Distribution of the Burrowing Shrimp Callichirus major (Say, 1818) (Decapoda, Callichiridae) in Preserved Populations of Southeastern Brazil. Thalassas 36, 333–342 (2020). https://doi.org/10.1007/s41208-020-00243-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41208-020-00243-7