Abstract
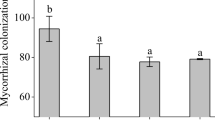
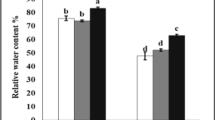
Arbuscular mycorrhizal (AM) fungi play an important role in improving the plant tolerance to salt stress. In the present study, we investigated the influence of AM fungi inoculation on various physiological, biochemical and nutritional aspects of pea grown under salt stress. The AM fungi inoculation successfully reduced the negative effects of salinity by improving the antioxidant enzyme system, a greater accumulation of compatible organic solutes, a higher content of photosynthetic pigment and a balanced uptake of nutrients, which resulted in higher growth and yield. Seed yield was found to be significantly higher by ~ 24, 40 and 54% in T2 (Rhizoglomus intraradices), T3 (Funneliformis mosseae and R. intraradices) and T4 (Rhizoglomus fasciculatum and Gigaspora sp.), respectively, as compared to nonmycorrhizal plants. Overall, a mixed application of R fasciculatum and Gigaspora sp. was superior to other mycorrhizal treatments, which can be attributed to specific compatibility relationships or functional complementarity that exists between symbionts.







Similar content being viewed by others
References
Abd_Allah EF, Hashem A, Alqarawi AA, Bahkali AH, Alwhibi MS (2015a) Enhancing growth performance and systemic acquired resistance of medicinal plant Sesbania sesban (L.) Merr using arbuscular mycorrhizal fungi under salt stress. Saudi J Bio Sci 22:274–283
Abd_Allah EF, Hashem A, Alqarawi AA, Alwhibi Mona S (2015b) Alleviation of adverse impact of salt in Phaseolus vulgaris L. by arbuscular mycorrhizal fungi. Pak J Bot 47:1167–1176
Abdel Latef AAH, Chaoxing H (2011) Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci Hort 127:228–233
Ahanger MA, Tyagi SR, Wani MR, Ahmad P (2014) Drought tolerance: role of organic osmolytes, growth regulators, and mineral nutrients. In: Ahmad P, Wani MR (eds) Physiological mechanisms and adaptation strategies in plants under changing environment, vol 1. Springer, New York, pp 25–55
Ahanger MA, Agarwal R, Tomar NS, Shrivastava M (2015) Potassium induces positive changes in nitrogen metabolism and antioxidant system of oat (Avena sativa L cultivar Kent). J Plant Interact 10:211–223
Ahmad P, Sharma S (2008) Salt stress and phyto-biochemical responses of plants—a review. Plant Soil Environ 54(3):89–99
Ahmad P, Jaleel CA, Sharma S (2010) Antioxidant defense system, lipid peroxidation, proline metabolizing enzymes, and biochemical activities in two Morus alba genotypes subjected to NaCl stress. Russian J Plant Physiol 57:509–517
Al-Khaliel AS (2010) Effect of salinity stress on mycorrhizal association and growth response of peanut infected by Glomus mosseae. Plant Soil Environ 56:318–324
Alqarawi AA, Abd Allah EF, Hashem A (2014) Alleviation of salt-induced adverse impact via mycorrhizal fungi in Ephedra aphylla Forssk. J Plant Interact 9:802–810
Amanifar S, Khodabandeloo M, Fard EM, Askari MS, Ashrafi M (2019) Alleviation of salt stress and changes in glycyrrhizin accumulation by arbuscular mycorrhiza in liquorice (Glycyrrhiza glabra) grown under salinity stress. Environ Exper Bot 160:25–34
Arnon DI (1949) Copper enzymes in isolated chloroplast polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Aroca R, Ruiz-Lozano JM, Zamarreño ÁM, Paz JA, García-Mina JM, Pozo MJ, López-Ráez JA (2013) Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J Plant Physiol 170:47–55
Chance B, Maehly AC (1955) Assay of catalase and peroxidase. Methods Enzymol 2:764–775
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F (1951) A colorimetric method for the estimation of sugar. Nature 168:167
Elhindi KM, El-Din AS, Elgorban AM (2017) The impact of arbuscular mycorrhizal fungi in mitigating salt-induced adverse effects in sweet basil (Ocimum basilicum L.). Saudi J Biol Sci 24:170–179
El-Tayeb MA (2005) Response of barley grains to the interactive effect of salinity and salicylic acid. Plant Growth Regul 45:215–224
Foyer CH, Descourvieres P, Kunert KJ (1994) Protection against oxygen radicals: an important defense mechanism studied in transgenic plants. Plant, Cell Environ 17:507–523
Grieve CM, Grattan SR (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307
Hashem A, Abd_Allah EF, Alqarawi AA, Aldubise A, Egamberdieva D, (2015) Arbuscular mycorrhizal fungi enhances salinity tolerance of Panicum turgidum Forssk by altering photosynthetic and antioxidant pathways. J Plant Interact 10:230–242
Hashem A, Alqarawi AA, Radhakrishnan R, Al-Arjani AB, Aldehaish HA, Egamberdieva D, Abd_Allah EF (2018) Arbuscular mycorrhizal fungi regulate the oxidative system, hormones and ionic equilibrium to trigger salt stress tolerance in Cucumis sativus L. Saudi J Biol Sci 25:1102–1114
Hossain MA, Fujita M (2010) Evidence for a role of exogenous glycine betaine and proline in antioxidant defense and methylglyoxal detoxification systems in mung bean seedlings under salt stress. Physiol Mol Biol Plants 16:19–29
Huang YM, Zou YN, Wu QS (2017) Alleviation of drought stress by mycorrhizas is related to increased root H2O2 efflux in trifoliate orange. Sci Rep 7:2335
Iqbal N, Umar S, Khan NA (2015) Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J Plant Physiol 178:84–91
Jakobsen I, Abbott LK, Robson AD (1992) External hyphae of vesicular-arbuscular mycorrhizal fungi associated with trifolium-subterraneum L. 1. Spread of hyphae and phosphorus inflow into roots. New Phytol 120:371–380
Kaya C, Ashraf M, Sonmez O, Aydemir S, Tuna AL, Cullu MA (2009) The influence of arbuscular mycorrhizal colonization on key growth parameters and fruit yield of pepper plants grown at high salinity. Sci Hortic 12:1–6
Khan MIR, Asgher M, Khan NA (2014) Alleviation of salt induced photosynthesis and growth inhibition by salicylic acid involves glycine betaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol Biol 80:67–74
Koca H, Bor M, Özdemir F, Türkan I (2007) The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ Exp Bot 60:344–351
Kohler J, Hernández JA, Caravaca F, Roldán A (2009) Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress. Environ Exp Bot 65:245–252
Koide RT, Kabir Z (2000) Extraradical hyphae of the mycorrhizal fungus Glomus intraradices can hydrolyse organic phosphate. New Phytol 148:511–517
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mo Y, Wang Y, Yang R, Zheng J, Liu C, Li H, Ma J, Zhang Y, Wei C, Zhang X (2016) Regulation of plant growth, photosynthesis, antioxidation and osmosis by an arbuscular mycorrhizal fungus in watermelon seedlings under well-watered and drought conditions. Front Plant Sci 7:644
Moore S, Stein WH (1948) Photometric ninhydrin method for use in the chromatography of amino acids. J Biol Chem 176:367–388
Mukherjee SP, Choudhari MA (1983) Implications of water stress induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58:116–170
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Negrão S, Schmöckel SM, Tester M (2017) Evaluating physiological responses of plants to salinity stress. Ann Bot 119:1–11
Papageorgion GC, Murata N (1995) The unusually strong stabilizing effects of glycine betaine on the structure and function of the oxygenevolving photosystem II complex. Photosynth Res 44:243–252
Parida SK, Das AB (2005) Salt tolerance and salinity effects on plants. Ecotoxicol Environ Saf 60:324–349
Parihar P, Singh S, Singh R, Singh VP, Prasad SM (2015) Effect of salinity stress on plants and its tolerance strategies: a review. Environ Sci Pollut Control Ser 22:4056–4075
Parihar M, Rakshit A, Singh HB, Rana K (2019) Diversity of arbuscular mycorrhizal fungi in alkaline soils of hot sub humid eco-region of Middle Gangetic Plains of India. Acta Agric Scand B 69:386–397
Qun HZ, Xing HC, Bin ZZ, Rong ZZ, Song WH (2007) Changes of antioxidative enzymes and cell membrane osmosis in tomato colonized by arbuscular mycorrhizae under NaCl stress. Coll Surf B Bioint 59:128–133
Rabie GH, Almadini AM (2005) Role of bioinoculants in development of salt-tolerance of Vicia faba plants under salinity stress. Afr J Biotechnol 4:210–222
Ruiz-Lozano JM, Porcel R, Azcón C, Aroca R (2012) Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: new challenges in physiological and molecular studies. J Exp Bot 63:4033–4044
Saxena A, Raghuwanshi R, Singh HB (2016) Elevation of defense network in chilli against Colletotrichum capsici by phyllospheric Trichoderma strain. J Plant Growth Regul 35:377–389
Sheng M, Tang M, Chan H, Yang B, Zhang F, Huang Y (2008) Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 18:287–296
Smart RE, Bingham GE (1974) Rapid estimates of relative water content. Plant physiology. Plant Physiol 53:258–260
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic press, New York
Smith FA, Jakobsen I, Smith SE (2000) Spatial differences in acquisition of soil phosphate between two arbuscular mycorrhizal fungi in symbiosis with Medicago truncatula. New Phytol 147:357–366
Tang M, Chen H, Huang JC, Tian ZQ (2009) AM fungi effects on the growth and physiology of Zea mays seedlings under diesel stress. Soil Biol Biochem 41:936–940
Wolf B (1982) A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun Soil Sci Plant Anal 13:1035–1059
Wright DP, Read DJ, Scholes JD (1998) Mycorrhizal sink strength influences whole plant carbon balance of Trifolium repens L. Plant, Cell Environ 21:881–891
Wu QS, Zou YN, He XH (2010) Contributions of arbuscular mycorrhizal fungi to growth, photosynthesis, root morphology and ionic balance of citrus seedlings under salt stress. Acta Physiol Plant 32:297–304
Wu N, Li Z, Wu F, Tang M (2016) Comparative photochemistry activity and antioxidant responses in male and female Populus cathayana cuttings inoculated with arbuscular mycorrhizal fungi under salt. Sci Rep 6:37663
Acknowledgements
This work was supported by the Rajiv Gandhi National Fellowship, funded by University Grants Commission (UGC) (Grant no: RGNF-2014-15-SC-RAJ-72082).
Author information
Authors and Affiliations
Contributions
MP and AR conceived and designed the study. MP, AR, KR and SSJ carried out the experiments. MP, KR and HSJ analysed the data. All authors contributed to data interpretation. MP, KR and RPM wrote the manuscript. AR and GT provided guidance on the whole study and improved the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Parihar, M., Rakshit, A., Rana, K. et al. Arbuscular mycorrhizal fungi mediated salt tolerance by regulating antioxidant enzyme system, photosynthetic pathways and ionic equilibrium in pea (Pisum sativum L.). BIOLOGIA FUTURA 71, 289–300 (2020). https://doi.org/10.1007/s42977-020-00037-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42977-020-00037-1