Abstract
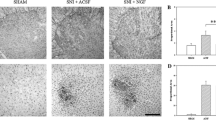
The sciatic nerve injury is accompanied by the activation of inflammatory processes in nerve fibers and the spinal cord lumbar segment, contributing to the development of neuropathic pain syndrome. In our study, ligation and transection of the sciatic nerve lead to a local inflammatory response, expressed in swelling and damage to nerve fibers and impaired innervation of peripheral tissues. At the same time, there is an increase in micro- and astroglial activity in the ventrolateral nucleus of the spinal cord lumbar segment (L4–L6), while motor neurons are encapsulated by microglia cells. At the same time, neurodegeneration of motor neurons is observed only during transection of the sciatic nerve. Thus, the results of this study indicate the active participation of the spinal cord motor nerve cells in the pathophysiological process after sciatic nerve injury.




Similar content being viewed by others
REFERENCES
Allodi, I., Udina, E., and Navarro, X., Specificity of peripheral nerve regeneration: interactions at the axon level, Prog. Neurobiol., 2012, vol. 98, p. 16.
Bennett, G.J. and Xie, Y.K., A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man, Pain, 1988, vol. 33, p. 87.
Brownjohn, P.W., and Ashton, J.C., Microglial encapsulation of motor neurons in models of neuropathic pain: a confound in pain assessment?, Eur. J. Pain, 2012, vol. 16, p. 459.
Camara-Lemarroy, C.R., Guzman-de la Garza, F.J., and Fernandez-Garza, N.E., Molecular inflammatory mediators in peripheral nerve degeneration and regeneration, Neuroimmunomodulation, 2010, vol. 17, p. 314.
Donegan, M., Kernisant, M., Cua, C., Jasmin, L., and Ohara, P.T., Satellite glial cell proliferation in the trigeminal ganglia after chronic constriction injury of the infraorbital nerve, Glia, 2013, vol. 61, p. 2000.
Hecke, O., Austin, S.K., Khan, R.A., Smith, B.H., and Torrance, N., Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain., 2014, vol. 155, p. 654.
Hu, P., Bembrick, A.L., Keay, K.A., and McLachlan, E.M., Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve, Brain Behav. Immun., 2007, vol. 21, p. 599.
Kiguchi, N., Maeda, T., Kobayashi, Y., Fukazawa, Y., and Kishioka, S., Macrophage inflammatory protein-1alpha mediates the development of neuropathic pain following peripheral nerve injury through interleukin-1beta up-regulation, Pain, 2010, vol. 149, p. 305.
Kobayashi, K., Imagama, S., Ohgomori, T., Hirano, K., Uchimura, K., Sakamoto, K., Hirakawa, A., Takeuchi, H., Suzumura, A., Ishiguro, N., and Kadomatsu, K., Minocycline selectively inhibits M1 polarization of microglia, Cell Death Dis., 2013, vol. 4, p. 1.
Korzhevskiǐ, D.E., Lentsman, M.V., Kirik, O.V., and Otellin, V.A., Morphological types of activated microglia in the hippocampus observed following transient total brain ischemia, Morfologiia, 2012, vol. 142, no. 2, p. 30.
Liao, B., Zhao, W., Beers, D.R., Henkel, J.S., and Appel, S.H., Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS, Exp. Neurol., 2012, vol. 237, p. 147.
Manzhulo, I.V.Ogurtsova, O.S., Tyrtyshnaia, A.A., and Dyuizen, I.V., Neuro-microglial interactions in the spinal centers of pain modulation in the neuropathic pain syndrome, Neurochem. J., 2017, vol. 11, p. 161.
Manzhulo, I.V., Ogurtsova, O.S., Kipryushina, Yu.O., Latyshev, N.A., Kasyanov, S.P., Dyuizen, I.V., and Tyrtyshnaia, A.A., Neuron-astrocyte interactions in spinal cord dorsal horn in neuropathic pain development and docosahexaenoic acid therapy, J. Neuroimmunol., 2016, vol. 298, p. 90.
Manzhulo, I.V., Ogurtsova, O.S., Lamash, N.E., Latyshev, N.A., Kasyanov, S.P., and Dyuizen, I.V., Analetic effect of docosahexaenoic acid is mediated by modulating the microglia activity in the dorsal root ganglia in a rat model of neuropathic pain, Acta Histochem., 2015, vol. 117, p. 659.
Mason, P., Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annu. Rev. Neurosci., 2001, vol. 24, p. 737.
Nakazato-Imasato, E. and Kurebayashi, Y., Pharmacological characteristics of the hind paw weight bearing difference induced by chronic constriction injury of the sciatic nerve in rats, Life Sci., 2009, vol. 84, p. 622.
Pannell, M., Labuz, D., Celik, M.Ö., Keye, J., Batra, A., Siegmund, B., and Machelska, H., Adoptive transfer of M2 macrophages reduces neuropathic pain via opioid peptides, J. Neuroinflamm., 2016, vol. 13, p. 262.
Qian, C., Tan, D., Wang, X., Li, L., Wen, J., Pan, M., Li, Y., Wu, W., and Guo, J., Peripheral nerve injury-induced astrocyte activation in spinal ventral horn contributes to nerve regeneration, Neural. Plast., 2018, vol. 2018, p. 8561704.
Schmued, L.C., Stowers, C.C., Scallet, A.C., and Xu, L., Fluoro-Jade C results in ultrahigh resolution and contrast labeling of degenerating neurons, Brain Res., 2005, vol. 1035, p. 24. rayama, R., Yamamoto, Y., Kishimoto, N., Tabata, M., Maruhama, K., Iida, S., and Sugimoto, T., Differential changes in neuronal excitability in the spinal dorsal horn after spinal nerve ligation in rats, Neurochem. Res., 2016, vol. 41, p. 2880.
Watkins, L.R., Goehler, L.E., Relton, J., Brewer, M.T., and Maier, S.F., Mechanisms of tumor necrosis factor-alpha (TNF-α) hyperalgesia, Brain Res., 1995, vol. 692, p. 244.
Funding
The present study was supported by the Russian Science Foundation (project no. 17‑74‑20006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for animal care and use have been followed. All procedures were approved by the Ethics Committee of the Zhirmunsky National Scientific Center for Marine Biology, Far Eastern Branch, Russian Academy of Sciences.
Rights and permissions
About this article
Cite this article
Starinets, A.A., Egorova, E.L., Tyrtyshnaia, A.A. et al. Micro- and Astroglia Activity in the Spinal Cord Ventrolateral Nucleus Sciatic Nerve Injury in Rats. Cell Tiss. Biol. 14, 263–269 (2020). https://doi.org/10.1134/S1990519X20040100
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X20040100