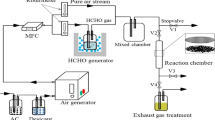
Abstract
High reduction temperature generally induces the agglomeration of supported noble metals. Howerve, we found that high temperature reduction did not induce Pd particles sintering but improved Pd dispersion. Multiple methods were further carried out to illuminate the abnormal phenomenon. The results indicated more surface oxygen defects and diffusion of Pd particles were simultaneously induced by high temperature reduction. During diffusion process of Pd particles, they were trapped by the oxygen defects because of the strong metal-support interaction, which led to improvement of Pd dispersion on the Pd/TiO2-450R catalyst. In addition, more surface oxygen vacancies on the Pd/TiO2-450R catalyst resulted in more active sites of H2O activation to form abundant surface OH groups which further enhanced adsorbed O2 activation and mobility, and then opening a more effective pathway for HCHO oxidation, which result in its high activity of Pd/TiO2-450R for ambient HCHO oxidation.








Similar content being viewed by others
References
Zhang L, Jiang Y, Chen B-B, Shi C, Li Y, Wang C, Han S, Pan S, Wang L, Meng X, Xiao F-S (2019) Exceptional activity for formaldehyde combustion using siliceous Beta zeolite as a catalyst support. Catal Today. https://doi.org/10.1016/j.cattod.2019.01.016
Sun XC, Lin J, Guan HL, Li L, Sun L, Wang YH, Miao S, Su Y, Wang XD (2018) Complete oxidation of formaldehyde over TiO2 supported subnanometer Rh catalyst at ambient temperature. Appl Catal B 226:575–584. https://doi.org/10.1016/j.apcatb.2018.01.011
de Falco G, Li W, Cimino S, Bandosz TJ (2018) Role of sulfur and nitrogen surface groups in adsorption of formaldehyde on nanoporous carbons. Carbon 138:283–291. https://doi.org/10.1016/j.carbon.2018.05.067
Zhu M, Muhammad Y, Hu P, Wang B, Wu Y, Sun X, Tong Z, Zhao Z (2018) Enhanced interfacial contact of dopamine bridged melamine-graphene/TiO2 nano-capsules for efficient photocatalytic degradation of gaseous formaldehyde. Appl Catal B 232:182–193. https://doi.org/10.1016/j.apcatb.2018.03.061
Fang RM, Huang HB, Ji J, He M, Feng QY, Zhan YJ, Leung DYC (2018) Efficient MnOx supported on coconut shell activated carbon for catalytic oxidation of indoor formaldehyde at room temperature. Chem Eng J 334:2050–2057. https://doi.org/10.1016/j.cej.2017.11.176
Quiroz Torres J, Royer S, Bellat JP, Giraudon JM, Lamonier JF (2013) Formaldehyde: catalytic oxidation as a promising soft way of elimination. Chemsuschem 6(4):578–592. https://doi.org/10.1002/cssc.201200809
Bai BY, Li JH (2014) Positive Effects of K+ Ions on Three-Dimensional Mesoporous Ag/Co3O4 Catalyst for HCHO Oxidation. ACS Catal 4(8):2753–2762. https://doi.org/10.1021/Cs5006663
Du X, Li C, Zhao L, Zhang J, Gao L, Sheng J, Yi Y, Chen J, Zeng G (2018) Promotional removal of HCHO from simulated flue gas over Mn–Fe oxides modified activated coke. Appl Catal B 232:37–48. https://doi.org/10.1016/j.apcatb.2018.03.034
Tang XF, Chen JL, Huang XM, Xu YD, Shen WJ (2008) Pt/MnOx–CeO2 catalysts for the complete oxidation of formaldehyde at ambient temperature. Appl Catal B 81(1–2):115–121. https://doi.org/10.1016/j.apcatb.2007.12.007
Tang XF, Li YG, Huang XM, Xu YD, Zhu HQ, Wang JG, Shen WJ (2006) MnOx–CeO2 mixed oxide catalysts for complete oxidation of formaldehyde: effect of preparation method and calcination temperature. Appl Catal B-Environ 62(3–4):265–273. https://doi.org/10.1016/j.apcatb.2005.08.004
Wang M, Zhang L, Huang W, Xiu T, Zhuang C, Shi J (2017) The catalytic oxidation removal of low-concentration HCHO at high space velocity by partially crystallized mesoporous MnOx. Chem Eng J 320:667–676. https://doi.org/10.1016/j.cej.2017.03.098
Liu F, Rong S, Zhang P, Gao L (2018) One-step synthesis of nanocarbon-decorated MnO2 with superior activity for indoor formaldehyde removal at room temperature. Appl Catal B 235:158–167. https://doi.org/10.1016/j.apcatb.2018.04.078
Zhu L, Wang J, Rong S, Wang H, Zhang P (2017) Cerium modified birnessite-type MnO2 for gaseous formaldehyde oxidation at low temperature. Appl Catal B 211:212–221. https://doi.org/10.1016/j.apcatb.2017.04.025
Fan Z, Shi J, Zhang Z, Chen M, Shangguan W (2018) Promotion effect of potassium carbonate on catalytic activity of Co3O4 for formaldehyde removal. J Chem Technol Biotechnol 93(12):3562–3568. https://doi.org/10.1002/jctb.5733
Wang HC, Guo WQ, Jiang Z, Yang R, Jiang Z, Pan Y, Shangguan WF (2018) New insight into the enhanced activity of ordered mesoporous nickel oxide in formaldehyde catalytic oxidation reactions. J Catal 361:370–383. https://doi.org/10.1016/j.jcat.2018.02.023
Bai BY, Arandiyan H, Li JH (2013) Comparison of the performance for oxidation of formaldehyde on nano-Co3O4, 2D-Co3O4, and 3D-Co3O4 catalysts. Appl Catal B 142:677–683. https://doi.org/10.1016/j.apcatb.2013.05.056
Yan Z, Xu Z, Cheng B, Jiang C (2017) Co3O4 nanorod-supported Pt with enhanced performance for catalytic HCHO oxidation at room temperature. Appl Surf Sci 404:426–434. https://doi.org/10.1016/j.apsusc.2017.02.010
Li YB, Chen XY, Wang CY, Zhang CB, He H (2018) Sodium enhances Ir/TiO2 activity for catalytic oxidation of formaldehyde at ambient temperature. ACS Catal 8(12):11377–11385. https://doi.org/10.1021/acscatal.8b03026
Ma C, Wang D, Xue W, Dou B, Wang H, Hao Z (2011) Investigation of formaldehyde oxidation over Co3O4−CeO2 and Au/Co3O4−CeO2 catalysts at room temperature: effective removal and determination of reaction mechanism. Environ Sci Technol 45(8):3628–3634. https://doi.org/10.1021/es104146v
Li YB, Zhang CB, He H, Zhang JH, Chen M (2016) Influence of alkali metals on Pd/TiO2 catalysts for catalytic oxidation of formaldehyde at room temperature. Catal Sci Technol 6(7):2289–2295. https://doi.org/10.1039/C5CY01521A
Zhang CB, Liu FD, Zhai YP, Ariga H, Yi N, Liu YC, Asakura K, Flytzani-Stephanopoulos M, He H (2012) Alkali-metal-promoted Pt/TiO2 opens a more efficient pathway to formaldehyde oxidation at ambient temperatures. Angew Chem Int Ed 51(38):9628–9632. https://doi.org/10.1002/anie.201202034
Zhai Y, Pierre D, Si R, Deng W, Ferrin P, Nilekar AU, Peng G, Herron JA, Bell DC, Saltsburg H, Mavrikakis M, Flytzani-Stephanopoulos M (2010) Alkali-stabilized Pt-OHx species catalyze low-temperature water-gas shift reactions. Science 329(5999):1633. https://doi.org/10.1126/science.1192449
Yang M, Li S, Wang Y, Herron JA, Xu Y, Allard LF, Lee S, Huang J, Mavrikakis M, Flytzani-Stephanopoulos M (2014) Catalytically active Au-O(OH)x- species stabilized by alkali ions on zeolites and mesoporous oxides. Science 346(6216):1498. https://doi.org/10.1126/science.1260526
Li Y, Zhang C, Ma J, Chen M, Deng H, He H (2017) High temperature reduction dramatically promotes Pd/TiO2 catalyst for ambient formaldehyde oxidation. Appl Catal B 217:560–569. https://doi.org/10.1016/j.apcatb.2017.06.023
Jones J, Xiong H, DeLaRiva AT, Peterson EJ, Pham H, Challa SR, Qi G, Oh S, Wiebenga MH, Pereira Hernández XI, Wang Y, Datye AK (2016) Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353(6295):150. https://doi.org/10.1126/science.aaf8800
Sanchez MG, Gazquez JL (1987) Oxygen vacancy model in strong metal-support interaction. J Catal 104(1):120–135. https://doi.org/10.1016/0021-9517(87)90342-3
Jiawei Wan WC, Jia C, Zheng L, Dong J, Xusheng Zheng Yu, Wang WY, Chen C, Peng Q, Wang D, Li Y (2018) Defect effects on TiO2 nanosheets: stabilizing single atomic site Au and promoting catalytic properties. Adv Mater 8:1705369–1705377. https://doi.org/10.1002/adma.201705369
Pan X, Yang M-Q, Fu X, Zhang N, Xu Y-J (2013) Defective TiO2 with oxygen vacancies: synthesis, properties and photocatalytic applications. Nanoscale 5:3601–3614. https://doi.org/10.1039/c3nr00476g
Pan J-M, Maschhoff BL, Diebold U, Madey TE (1992) Interaction of water, oxygen, and hydrogen with TiO2(110) surfaces having different defect densities. J Vac Sci Technol A 10:7. https://doi.org/10.1116/1.577986
Li Y, Xu B, Fan Y, Feng N, Qiu A, He JMJ, Yang H, Chen Y (2004) The effect of titania polymorph on the strong metal-support interaction of Pd/TiO2 catalysts and their application in the liquid phase selective hydrogenation of long chain alkadienes. J Mol Catal A 216(1):107–114. https://doi.org/10.1016/j.molcata.2004.02.007
Bromiley GD, Shiryaev AA (2006) Neutron irradiation and post-irradiation annealing of rutile (TiO2−x): effect on hydrogen incorporation and optical absorption. Phys Chem Miner 33(6):426–434. https://doi.org/10.1007/s00269-006-0087-9
Wang L-Q, Baer DR, Engelhard MH, Shultz AN (1995) The adsorption of liquid and vapor water on TiO2(110) surfaces: the role of defects. Surf Sci 344(3):237–250. https://doi.org/10.1016/0039-6028(95)00859-4
Dutta PK, Ginwalla A, Hogg B, Patton BR, Chwieroth B, Liang Z, Gouma P, Mills M, Akbar S (1999) Interaction of carbon monoxide with anatase surfaces at high temperatures: optimization of a carbon monoxide sensor. J Phys Chem B 103(21):4412–4422. https://doi.org/10.1021/jp9844718
Zhang CB, Li YB, Wang YF, He H (2014) Sodium-promoted Pd/TiO2 for catalytic oxidation of formaldehyde at ambient temperature. Environ Sci Technol 48(10):5816–5822. https://doi.org/10.1021/Es4056627
Li J, Zhang M, Guan Z, Li Q, He C, Yang J (2017) Synergistic effect of surface and bulk single-electron-trapped oxygen vacancy of TiO2 in the photocatalytic reduction of CO2. Appl Catal B 206:300–307. https://doi.org/10.1016/j.apcatb.2017.01.025
Brookes IM, Muryn CA, Thornton G (2001) Imaging water dissociation on TiO2(110). Phys Rev Lett 87(26):266103. https://doi.org/10.1103/PhysRevLett.87.266103
Schaub R, Thostrup P, Lopez N, Lægsgaard E, Stensgaard I, Nørskov JK, Besenbacher F (2001) Oxygen vacancies as active sites for water dissociation on rutile TiO2(110). Phys Rev Lett 87(26):266104. https://doi.org/10.1103/PhysRevLett.87.266104
Zeng L, Song WL, Li MH, Zeng DW, Xie CS (2014) Catalytic oxidation of formaldehyde on surface of HTiO2/HCTiO2 without light illumination at room temperature. Appl Catal B 147:490–498. https://doi.org/10.1016/j.apcatb.2013.09.013
Tauster SJ, Fung SC, Garten RL (1978) Strong metal-support interactions Group 8 Noble metals supported on TiO2. J Am Chem Soc 100(1):170–175. https://doi.org/10.1021/Ja00469a029
Vannice MA, Twu CC, Moon SH (1983) SMSI effects on CO adsorption and hydrogenation on Pt catalysts: I. Infrared spectra of adsorbed CO prior to and during reaction conditions. J Catal 79(1):70–80. https://doi.org/10.1016/0021-9517(83)90290-7
Benvenutti EV, Franken L, Moro CC, Davanzo CU (1999) FTIR study of hydrogen and carbon monoxide adsorption on Pt/TiO2, Pt/ZrO2, and Pt/Al2O3. Langmuir 15(23):8140–8146. https://doi.org/10.1021/La990195s
Sheu LL, Sachtler WMH (1993) Detection by CO-FTIR of incipient metal—support interaction in Rh/SiO2 and Pd/TiO2. J Mol Catal 81(2):267–278. https://doi.org/10.1016/0304-5102(93)80011-I
Sá J, Bernardi J, Anderson J (2007) Imaging of low temperature induced SMSI on Pd/TiO2 catalysts. Catal Lett 114(1–2):91–95. https://doi.org/10.1007/s10562-007-9049-1
Zhu HQ, Qin ZF, Shan WJ, Shen WJ, Wang JW (2005) Low-temperature oxidation of CO over Pd/CeO2–TiO2 catalysts with different pretreatments. J Catal 233(1):41–50. https://doi.org/10.1016/j.jcat.2005.04.033
Zhang Z, Bondarchuk O, Kay BD, White JM, Dohnalek Z (2006) Imaging water dissociation on TiO2 (110): evidence for inequivalent geminate OH groups. J Phys Chem B 110(43):21840–21845. https://doi.org/10.1021/Jp063619h
Ojifinni RA, Froemming NS, Gong J, Pan M, Kim TS, White JM, Henkelman G, Mullins CB (2008) Water-enhanced low-temperature CO oxidation and isotope effects on atomic oxygen-covered Au(111). J Am Chem Soc 130(21):6801–6812. https://doi.org/10.1021/ja800351j
Bongiorno A, Landman U (2005) Water-enhanced catalysis of CO oxidation on free and supported gold nanoclusters. Phys Rev Lett 95(106102):1–4. https://doi.org/10.1103/PhysRevLett.95.106102
Kim TS, Gong J, Ojifinni RA, White JM, Mullins CB (2006) Water activated by atomic oxygen on Au(111) to oxidize CO at low temperatures. J Am Chem Soc 128(19):6282–6283. https://doi.org/10.1021/ja058263m
Huang HB, Leung DYC (2011) Complete oxidation of formaldehyde at room temperature using TiO2 supported metallic Pd nanoparticles. ACS Catal 1(4):348–354. https://doi.org/10.1021/cs200023p
Liu LM, McAllister B, Ye HQ, Hu P (2006) Identifying an O2 supply pathway in CO oxidation on Au/TiO2 (110): a density functional theory study on the intrinsic role of water. J Am Chem Soc 128(12):4017–4022. https://doi.org/10.1021/Ja056801p
Ammal SC, Heyden A (2014) Water-gas shift catalysis at corner atoms of Pt clusters in contact with a TiO2 (110) support surface. ACS Catal 4(10):3654–3662. https://doi.org/10.1021/cs5009706
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21707136) and Natural Science Foundation of Fujian Province, China (Grant No. 2018J05027).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Y., Wang, C., Zhang, C. et al. Formaldehyde Oxidation on Pd/TiO2 Catalysts at Room Temperature: The Effects of Surface Oxygen Vacancies. Top Catal 63, 810–816 (2020). https://doi.org/10.1007/s11244-020-01349-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-020-01349-1