Abstract
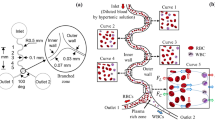
Magnetophoretic separation has gained much attention in recent years due to its easy application and low-cost fabrication compared to other active particle separation techniques. Due to the different properties of white blood cells (WBCs) and red blood cells (RBCs), it is possible to manipulate and separate them using a magnetic field. In this paper, a simple microfluidic device is proposed to fractionate WBCs and RBCs from whole blood using magnetophoretic force applied by Halbach array of three permanent magnets. Plasma streams containing WBCs and RBCs enter a simple microchip fabricated by PDMS. Permanent magnets apply positive and negative magnetophoretic forces to the RBCs and WBCs, respectively. Two cladding streams containing blood plasma are used to concentrate the cells in the magnetophoretic area. A wide range of inlet velocities and different distances of magnets from the channel (d) are investigated. It is demonstrated that the volume flow rate of core, and cladding streams, total flow rate and the distance between magnets and microchannel affect the separation efficiency individually. The results reveal that d = 0.1, 0.2, 0.3, 0.4, and 0.5 mm may lead to complete separation when core and cladding flow rates are 1 and 7 μl/h, respectively.















Similar content being viewed by others
References
Dalili A, Samiei E, Hoorfar M (2019) A review of sorting, separation and isolation of cells and microbeads for biomedical applications: microfluidic approaches. Analyst 144(1):87–113
Sajeesh P, Sen AK (2014) Particle separation and sorting in microfluidic devices: a review. Microfluid Nanofluid 17(1):1–52
Yan S, Tan SH, Li Y, Tang S, Teo AJ, Zhang J, Zhao Q, Yuan D, Sluyter R, Nguyen N-T (2018) A portable, hand-powered microfluidic device for sorting of biological particles. Microfluid Nanofluid 22(1):8
Chen J, Chen D, Yuan T, Chen X, Xie Y, Fu H, Cui D, Fan X, Oo MKK (2014) Blood plasma separation microfluidic chip with gradual filtration. Microelectron Eng 128:36–41
Kang Y-T, Doh I, Byun J, Chang HJ, Cho Y-H (2017) Label-free rapid viable enrichment of circulating tumor cell by photosensitive polymer-based microfilter device. Theranostics 7(13):3179
Liu C, Mauk M, Gross R, Bushman FD, Edelstein PH, Collman RG, Bau HH (2013) Membrane-based, sedimentation-assisted plasma separator for point-of-care applications. Anal Chem 85(21):10463–10470
Amini H, Lee W, Di Carlo D (2014) Inertial microfluidic physics. Lab Chip 14(15):2739–2761
Di Carlo D, Irimia D, Tompkins RG, Toner M (2007) Continuous inertial focusing, ordering, and separation of particles in microchannels. Proc Natl Acad Sci 104(48):18892–18897
Martel JM, Toner M (2012) Inertial focusing dynamics in spiral microchannels. Phys Fluids 24(3):032001
Rafeie M, Zhang J, Asadnia M, Li W, Warkiani ME (2016) Multiplexing slanted spiral microchannels for ultra-fast blood plasma separation. Lab Chip 16(15):2791–2802
Dincau BM, Aghilinejad A, Hammersley T, Chen X, Kim J-H (2018) Deterministic lateral displacement (DLD) in the high Reynolds number regime: high-throughput and dynamic separation characteristics. Microfluid Nanofluid 22(6):59
Holm SH, Beech JP, Barrett MP, Tegenfeldt JO (2011) Separation of parasites from human blood using deterministic lateral displacement. Lab Chip 11(7):1326–1332
Huang LR, Cox EC, Austin RH, Sturm JC (2004) Continuous particle separation through deterministic lateral displacement. Science 304(5673):987–990
Loutherback K, D’Silva J, Liu L, Wu A, Austin RH, Sturm JC (2012) Deterministic separation of cancer cells from blood at 10 mL/min. AIP Adv 2(4):042107
Jain A, Posner JD (2008) Particle dispersion and separation resolution of pinched flow fractionation. Anal Chem 80(5):1641–1648
Vig AL, Kristensen A (2008) Separation enhancement in pinched flow fractionation. Appl Phys Lett 93(20):203507
Yamada M, Nakashima M, Seki M (2004) Pinched flow fractionation: continuous size separation of particles utilizing a laminar flow profile in a pinched microchannel. Anal Chem 76(18):5465–5471
Pethig R (2010) Dielectrophoresis: status of the theory, technology, and applications. Biomicrofluidics 4(2):022811
Shafiee H, Caldwell JL, Sano MB, Davalos RV (2009) Contactless dielectrophoresis: a new technique for cell manipulation. Biomed Microdev 11(5):997
Zhu H, Lin X, Su Y, Dong H, Wu J (2015) Bioelectronics. Screen-printed microfluidic dielectrophoresis chip for cell separation. Biosens Bioelectron 63:371–378
Das D, Biswas K, Das S (2014) Physics. A microfluidic device for continuous manipulation of biological cells using dielectrophoresis. Med Eng Phys 36(6):726–731
Vykoukal J, Vykoukal DM, Freyberg S, Alt EU, Gascoyne PR (2008) Enrichment of putative stem cells from adipose tissue using dielectrophoretic field-flow fractionation. Lab Chip 8(8):1386–1393
Yunus NAM, Nili H, Green NG (2013) Continuous separation of colloidal particles using dielectrophoresis. Electrophoresis 34(7):969–978
Ding X, Li P, Lin S-CS, Stratton ZS, Nama N, Guo F, Slotcavage D, Mao X, Shi J, Costanzo F (2013) Surface acoustic wave microfluidics. Lab Chip 13(18):3626–3649
Petersson F, Åberg L, Swärd-Nilsson A-M, Laurell T (2007) Free flow acoustophoresis: microfluidic-based mode of particle and cell separation. Anal Chem 79(14):5117–5123
Shi J, Huang H, Stratton Z, Huang Y, Huang T (2009) Continuous particle separation in a microfluidic channel via standing surface acoustic waves (SSAW). Lab Chip 9(23):3354–3359
Chen Y, Wu M, Ren L, Liu J, Whitley PH, Wang L, Huang TJ (2016) High-throughput acoustic separation of platelets from whole blood. Lab Chip 16(18):3466–3472
Han K-H, Bruno Frazier A (2004) Continuous magnetophoretic separation of blood cells in microdevice format. J Appl Phys 96(10):5797–5802
Pamme N, Manz A (2004) On-chip free-flow magnetophoresis: continuous flow separation of magnetic particles and agglomerates. Anal Chem 76(24):7250–7256
Han K-H, Frazier AB (2005) Diamagnetic capture mode magnetophoretic microseparator for blood cells. J Microelectromech Syst 14(6):1422–1431
Han K-H, Frazier AB (2005) A microfluidic system for continuous magnetophoretic separation of suspended cells using their native magnetic properties. Proc Nanotechol 1:187–190
Tzirtzilakis E (2005) A mathematical model for blood flow in magnetic field. Phys Fluids 17(7):077103
Furlani EP (2007) Magnetophoretic separation of blood cells at the microscale. J Phys D Appl Phys 40(5):1313
Adams JD, Kim U, Soh HT (2008) Multitarget magnetic activated cell sorter. Proc Natl Acad Sci 105(47):18165–18170
Gijs MA, Lacharme F, Lehmann U (2010) Microfluidic applications of magnetic particles for biological analysis and catalysis. Chem Rev 110(3):1518–1563
Seo H-K, Kim H-O, Kim Y-J (2010) Hydrodynamics and magnetophoresis based hybrid blood cell sorter. In: 10th IEEE international conference on nanotechnology. IEEE, pp 911–914
Seo H-K, Kim Y-H, Kim H-O, Kim Y-J (2010) Hybrid cell sorters for on-chip cell separation by hydrodynamics and magnetophoresis. J Micromech Microeng 20(9):095019
Zhu T, Marrero F, Mao L (2010) Continuous separation of non-magnetic particles inside ferrofluids. Microfluid Nanofluid 9(4–5):1003–1009
Baek MK, Choi HS, Lee KS, Park IH (2011) Numerical analysis for magnetophoretic separation of blood cells in fluid and magnetic field. IEEE Trans Appl Supercond 22(3):4401604
Forbes TP, Forry SP (2012) Microfluidic magnetophoretic separations of immunomagnetically labeled rare mammalian cells. Lab Chip 12(8):1471–1479
Mizuno M, Yamada M, Mitamura R, Ike K, Toyama K, Seki M (2013) Magnetophoresis-integrated hydrodynamic filtration system for size-and surface marker-based two-dimensional cell sorting. Anal Chem 85(16):7666–7673
Nam J, Huang H, Lim H, Lim C, Shin S (2013) Magnetic separation of malaria-infected red blood cells in various developmental stages. Anal Chem 85(15):7316–7323
Zhu T, Cheng R, Liu Y, He J, Mao L (2014) Combining positive and negative magnetophoreses to separate particles of different magnetic properties. Microfluid Nanofluid 17(6):973–982
Fateen S-EK, Magdy M (2015) Design. Three dimensional simulation of negative-magnetophoretic filtration of non-magnetic nanoparticles. Chem Eng Res Des 95:69–78
Hejazian M, Li W, Nguyen N-T (2015) Lab on a chip for continuous-flow magnetic cell separation. Lab Chip 15(4):959–970
Zhou Y, Kumar DT, Lu X, Kale A, DuBose J, Song Y, Wang J, Li D, Xuan X (2015) Simultaneous diamagnetic and magnetic particle trapping in ferrofluid microflows via a single permanent magnet. Biomicrofluidics 9(4):044102
Hejazian M, Nguyen N-T (2016) Magnetofluidic concentration and separation of non-magnetic particles using two magnet arrays. Biomicrofluidics 10(4):044103
Kim MJ, Lee DJ, Youn JR, Song YS (2016) Two step label free particle separation in a microfluidic system using elasto-inertial focusing and magnetophoresis. RSC Adv 6(38):32090–32097
Zhang J, Yan S, Yuan D, Zhao Q, Tan SH, Nguyen N-T, Li W (2016) A novel viscoelastic-based ferrofluid for continuous sheathless microfluidic separation of nonmagnetic microparticles. Lab Chip 16(20):3947–3956
Zhou Y, Xuan X (2016) Diamagnetic particle separation by shape in ferrofluids. Appl Phys Lett 109(10):102405
Chen Q, Li D, Lin J, Wang M, Xuan X (2017) Simultaneous separation and washing of nonmagnetic particles in an inertial ferrofluid/water coflow. Anal Chem 89(12):6915–6920
Tarn MD, Pamme N (2017) On-chip magnetic particle-based immunoassays using multilaminar flow for clinical diagnostics. Microchip diagnostics. Springer, New York, pp 69–83
Zhao W, Cheng R, Jenkins BD, Zhu T, Okonkwo NE, Jones CE, Davis MB, Kavuri SK, Hao Z, Schroeder C (2017) Label-free ferrohydrodynamic cell separation of circulating tumor cells. Lab Chip 17(18):3097–3111
Zhao W, Cheng R, Lim SH, Miller JR, Zhang W, Tang W, Xie J, Mao L (2017) Biocompatible and label-free separation of cancer cells from cell culture lines from white blood cells in ferrofluids. Lab Chip 17(13):2243–2255
Alnaimat F, Dagher S, Mathew B, Hilal-Alnqbi A, Khashan S (2018) Microfluidics based magnetophoresis: a review. Chem Rec 18(11):1596–1612
Cardoso VF, Miranda D, Botelho G, Minas G, Lanceros-Méndez S (2018) Highly effective clean-up of magnetic nanoparticles using microfluidic technology. Sens. Chem. Actuat. B 255:2384–2391
Munaz A, Shiddiky MJ, Nguyen N-T (2018) Recent advances and current challenges in magnetophoresis based micro magnetofluidics. Biomicrofluidics 12(3):031501
Munaz A, Shiddiky MJ, Nguyen N-T (2018) Chemical AB. Magnetophoretic separation of diamagnetic particles through parallel ferrofluid streams. Sens Chem Actuat B 275:459–469
Oh S, Jung SH, Seo H, Min M-K, Kim B, Hahn YK, Kang JH, Choi S (2018) Magnetic activated cell sorting (MACS) pipette tip for immunomagnetic bacteria separation. Sens Chem Actuat B 272:324–330
Wu J, Cui Y, Xuan S, Gong X (2018) Nanofluidics. 3D-printed microfluidic manipulation device integrated with magnetic array. Microfluidics and Nanofluidics 22(9):103
Lin S, Zhi X, Chen D, Xia F, Shen Y, Niu J, Huang S, Song J, Miao J, Cui D (2019) A flyover style microfluidic chip for highly purified magnetic cell separation. Biosens Bioelectron 129:175–181
Watarai H, Namba M (2002) Capillary magnetophoresis of human blood cells and their magnetophoretic trapping in a flow system. J Chromatogr A 961(1):3–8
Zborowski M, Ostera GR, Moore LR, Milliron S, Chalmers JJ, Schechter AN (2003) Red blood cell magnetophoresis. Biophys J 84(4):2638–2645
Vanderlinde J (2006) Classical electromagnetic theory. Springer, New York, p 145
Cheng R, Zhu T, Mao L (2014) Three-dimensional and analytical modeling of microfluidic particle transport in magnetic fluids. Microfluid Nanofluid 16(6):1143–1154
He Y, Luo L, Huang S (2019) Magnetic manipulation on the unlabeled nonmagnetic particles. Int J Mod Phys B 33(07):1950047
Bayareh M, Nazemi Ashani M, Usefian A (2020) Active and passive micromixers: a comprehensive review. Chem Eng Process Process Intensif 147:107771. https://doi.org/10.1016/j.cep.2019.107771
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shiriny, A., Bayareh, M. On magnetophoretic separation of blood cells using Halbach array of magnets. Meccanica 55, 1903–1916 (2020). https://doi.org/10.1007/s11012-020-01225-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11012-020-01225-y