Abstract
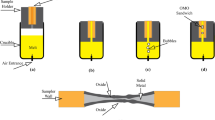
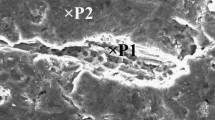
The entrainment of surface oxide films in the melt leads to the formation of double oxide defects in the casting parts. Oxide/metal/oxide (OMO) sandwich technique is a method for physical simulation of the formation of surface oxide film. In this method, air bubbles are artificially introduced into the melt in order to trap two adjacent bubbles and extract the interface between them. In this study, to prepare OMO samples, air bubbles were blown with a pressure of 0.8 atm. into the mold filled with Al melt containing 3, 5 and 7 wt% of Zn. Using scanning electron microscopy and energy-dispersive X-ray spectroscopy, characteristics of dynamically formed oxide films such as morphology and film thickness were investigated. Results showed that in higher zinc content, more cracks appear on the oxide films. Thickness of the oxide films in Al–Zn alloys was estimated to be 95–1070 nm. According to the measurement performed on the folds, adding more than 3% zinc caused a reduction in the oxide film thickness. Thermodynamics suggests the presence of spinel phase along with aluminum oxide at 700 °C which is in agreement with energy-dispersive X-ray spectroscopy’s results. The presence of the spinel phase at the interface is the reason behind the reduction in the thickness of the oxide film.
Graphic Abstract














Similar content being viewed by others
References
E.N. Coker, The oxidation of aluminum at high temperature studied by thermogravimetric analysis and differential scanning calorimetry. Advanced Materials Laboratory, Sandia National Laboratories (2013). https://doi.org/10.2172/1096501
M. Drouzy, C. Mascré, Metall. Rev. 14(1), 25–46 (1969)
S.J. Bonner, J.A. Taylor, J.Y. Yao, M.A. Rhamdhani, Oxidation of commercial purity aluminum melts: an experimental study, in Light Metals, The Minerals, Metals and Materials Series, ed. by B.A. Sadler (2016), pp. 993–997
J.M. Park, Behaviors of biofilms in A356 alloy during solidification: developing observation techniques with 3D micro X-ray tomography. Ph.D. Dissertation, School of Metallurgy and Materials College of Engineering, The University of Birmingham, 2009
P.K. Yuen, Effects of strontium on the oxidation of molten aluminum alloys containing silicon and magnesium. Ph.D. Dissertation, McGill University, 2001
S.J. Bonner, A microstructural and kinetic study of molten aluminium oxidation in relation to dross formation. Ph.D. dissertation, School of Mechanical and Mining Engineering, The University of Queensland, 2015
G. Wightman, D.J. Fray, Metall. Trans. B 14, 625–663 (1983)
J. Campbell, Complete Casting Handbook Metal Casting Processes, Metallurgy, Techniques and Design (Elsevier Butterworth-Heinemann, Oxford, 2015)
S.A. Azarmehr, M. Divandari, H. Arabi, Mater. Sci. Technol. 28(11), 1295–1300 (2012)
J. Campbell, J. Mater. Sci. 51(1), 96–106 (2015)
M. Divandari, J. Campbell, Trans. AFS 01–048, 8–10 (2001)
J. Campbell, Int. Metalcast. 1, 7–20 (2007). https://doi.org/10.1007/BF03355414
C. Vian, Int. Metalcast. 8, 59–60 (2014). https://doi.org/10.1007/BF03355573
C. Lee, T. So, K. Shin, Inter. Metalcast. 13, 880–889 (2019). https://doi.org/10.1007/s40962-019-00307-2
D. Dispinar, J. Campbell, Mater. Sci. Eng. A 528(10), 3860–3865 (2011)
J. Campbell, Inter. Metalcast. 6, 7–18 (2012). https://doi.org/10.1007/BF03355529
M. Divandari, J. Campbell, Alum. Trans. 2(2), 233–238 (2000)
M. Divandari, Mechanisms of bubble damage in castings. Ph.D. dissertation, School of Metallurgy and Materials, The University of Birmingham, 2001
M. Divandari, J. Campbell, Int. J. Cast Met. Res. 17(3), 182–187 (2004)
M. Divandari, M. Mehrabian, J. Mat. Sci. Eng. 14(3), 34–47 (2017)
M.M. Jalilvand, N.T. Bagh, M. Akbarifar, A. Divandari, Int. Metalcast. (2019). https://doi.org/10.1007/s40962-019-00395-0
I. Haginoya, T. Fukusako, IMONO 54, 664–669 (1982). https://doi.org/10.11279/imono.54.10-664
W. Kahl, E. Fromm, Metall. Mater. Trans. B 16, 47–51 (1985)
J. Liu, Q. Wang, Y. Qi, Acta Mater. 164, 673–682 (2019)
M. Divandari, J. Campbell, Int. J. Cast Metal Res. 18(3), 16–21 (2005)
M.M. Jalilvand, M. Akbarifar, M. Divandari, H. Saghafian, J. Magnes, Alloy 8(1), 219–230 (2020)
Y. Waseda, K.T. Jacob, T. Tsuchiya, S. Tamaki, J. Mater. Sci. Lett. 33(8), 940–945 (1978)
M.G.C. Cox, B. Mcenaney, V.D. Scott, Nat. Phys. Sci. 237, 140–142 (1972)
M.G.C. Cox, B. Mcenaney, V.D. Scott, Philos. Mag. 26(4), 839–851 (1972)
X. Jhou, H. Habazaki, K. Shimizu, P. Skeldon, G.E. Thompson, G.C. Wood, Corros. Sci. 38(9), 1563–1577 (1996)
E.M. Hinton, The oxidation of liquid aluminium and the potential for oxides in grain refinement of aluminium alloys. Ph.D. dissertation, University of Birmingham, 2014
C.W. Bale, E. Bélisle, P. Chartrand, Comput. Coupl. Phase Diagr. Thermochem. 54, 35–53 (2016)
G.M. Scamans, E.P. Butler, Met. Trans. A 6, 2055–2063 (1975)
M. Syvertsen, Oxide skin strength on molten AA5XXX aluminum alloy-effect of beryllium and alternatives, in Light Metals, (2017) pp. 1451–1455. https://doi.org/10.1007/978-3-319-0_173
S. Saha, C. Ravindran, Inter. Metalcast. 9, 39–48 (2015). https://doi.org/10.1007/BF0335603
Acknowledgements
We would like to express our sincere gratitude to Professor John Campbell for his valuable comments on this work. Financial support of deputy of research of Iran University of Science and Technology and the Cellular and Porous Materials Laboratory of IUST is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taheri Bagh, N., Divandari, M., Shahmiri, M. et al. Characteristics of Dynamically Formed Oxide Films in Al–Zn Melt. Inter Metalcast 15, 747–762 (2021). https://doi.org/10.1007/s40962-020-00501-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40962-020-00501-7