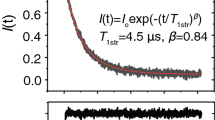
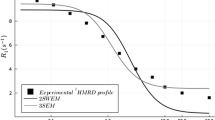
Abstract
A probability distribution of rate constants contained within an exponential-like saturation recovery (SR) electron paramagnetic resonance signal can be constructed using stretched exponential function fitting parameters. Previously (Stein et al. Appl. Magn. Reson. 2019.), application of this method was limited to the case where only one relaxation process, namely spin–lattice relaxations due to the rotational diffusion of the spin labels in the intact eye-lens membranes, contributed to an exponential-like SR signal. These conditions were achieved for thoroughly deoxygenated samples. Here, the case is described where the second relaxation process, namely Heisenberg exchange between the spin label and molecular oxygen that occurs during bimolecular collisions, contributes to the decay of SR signals. We have further developed the theory for application of stretched exponential function to analyze SR signals involving these two processes. This new approach allows separation of stretched exponential parameters, namely characteristic stretched rates and heterogeneity parameters for both processes. Knowing these parameters allowed us to separately construct the probability distributions of spin–lattice relaxation rates determined by the rotational diffusion of spin labels and the distribution of relaxations induced strictly by collisions with molecular oxygen. The later distribution is determined by the distribution of oxygen diffusion concentration products within the membrane, which forms a sensitive new way to describe membrane fluidity and heterogeneity. This method was validated in silico and by fitting SR signals from spin-labeled intact nuclear fiber cell plasma membranes extracted from porcine eye lenses equilibrated with different fractions of air.








Similar content being viewed by others
References
W.K. Subczynski, J.S. Hyde, A. Kusumi, Biochemistry 30, 8578 (1991)
R.J. Pace, S.I. Chan, J. Chem. Phys. 76, 4217 (1982)
C. Altenbach, D.A. Greenhalgh, H.G. Khorana, W.L. Hubbell, Proc. Natl. Acad. Sci. USA 91, 1667 (1994)
A. Kusumi, W.K. Subczynski, J.S. Hyde, Proc. Natl. Acad. Sci. USA 79, 1854 (1982)
W.K. Subczynski, J.S. Hyde, A. Kusumi, Proc. Natl. Acad. Sci. USA 86, 4474 (1989)
J. Widomska, M. Raguz, W.K. Subczynski, Biochim. Biophys. Acta Biomembr. 1768, 2635 (2007)
W.K. Subczynski, J.S. Hyde, Adv. Exp. Med. Biol. 454, 399–408 (1998)
W.K. Subczynski, L.E. Hopwood, J.S. Hyde, J. Gen. Physiol. 100, 69 (1992)
M. Raguz, L. Mainali, W.J. O’Brien, W.K. Subczynski, Exp. Eye Res. 132, 78 (2015)
W.K. Subczynski, H.M. Swartz, Biol. Magn. Reson. 23, 229–282 (2005)
W.K. Subczynski, J. Widomska, L. Mainali, Adv. Exp. Med. Biol. 977, 27–34 (2017)
I. Ashikawa, J.J. Yin, W.K. Subczynski, J.S. Hyde, T. Kouyama, A. Kusumi, Biochemistry 33, 4947 (1994)
K. Kawasaki, J.J. Yin, W.K. Subczynski, J.S. Hyde, A. Kusumi, Biophys. J. 80, 738 (2001)
M. Raguz, L. Mainali, W.J. O’Brien, W.K. Subczynski, Exp. Eye Res. 120, 138 (2014)
W.K. Subczynski, A. Wisniewska, J.S. Hyde, A. Kusumi, Biophys. J. 92, 1573 (2007)
M. Raguz, L. Mainali, J. Widomska, W.K. Subczynski, Biochim. Biophys. Acta Biomembr. 1808, 1072 (2011)
W.K. Subczynski, M. Raguz, J. Widomska, Methods Mol. Biol. 606, 247 (2010)
M. Raguz, L. Mainali, J. Widomska, W.K. Subczynski, Chem. Phys. Lipids 164, 819 (2011)
L. Mainali, M. Raguz, W.K. Subczynski, J. Phys. Chem. B 117, 8994 (2013)
W.K. Subczynski, M. Raguz, J. Widomska, L. Mainali, A. Konovalov, J. Membr. Biol. 245, 51 (2012)
L. Mainali, M. Pasenkiewicz-Gierula, W.K. Subczynski, Curr. Eye Res. 45, 162 (2020)
J. Widomska, W.K. Subczynski, L. Mainali, M. Raguz, Cell Biochem. Biophys. 75, 387 (2017)
C. Mailer, R.D. Nielsen, B.H. Robinson, J. Phys. Chem. A 109, 4049 (2005)
D. Marsh, J. Magn. Reson. 290, 38 (2018)
L. Mainali, J.B. Feix, J.S. Hyde, W.K. Subczynski, J. Magn. Reson. 212, 418 (2011)
L. Mainali, J.S. Hyde, W.K. Subczynski, J. Magn. Reson. 226, 35 (2013)
B.H. Robinson, D.A. Haas, C. Mailer, Science 263, 490 (1994)
W.K. Subczynski, J.S. Hyde, BBA Biomembr. 643, 283 (1981)
L. Huang, V. Grami, Y. Marrero, D. Tang, M.C. Yappert, V. Rasi, D. Borchman, Investig. Ophthalmol. Vis. Sci. 46, 1682 (2005)
C.A. Paterson, J. Zeng, Z. Husseini, D. Borchman, N.A. Delamere, D. Garland, J. Jimenez-Asensio, Curr. Eye Res. 16, 333 (1997)
S. Bassnett, Y. Shi, G.F.J.M. Vrensen, Philos. Trans. R. Soc. B Biol. Sci. 366, 1250 (2011)
T. Gonen, Y. Cheng, J. Kistler, T. Walz, J. Mol. Biol. 342, 1337 (2004)
J. Kistler, S. Bullivant, FEBS Lett. 111, 73 (1980)
V. Vidová, J. Pól, M. Volný, P. Novák, V. Havlícek, S.K. Wiedmer, J.M. Holopainen, J. Lipid Res. 51, 2295 (2010)
R.J.W. Truscott, Ophthalmic Res. 32, 185 (2000)
M.C. Yappert, M. Rujoi, D. Borchman, I. Vorobyov, R. Estrada, Exp. Eye Res. 76, 725 (2003)
D. Borchman, W.C. Byrdwell, M.C. Yappert, Investig. Ophthalmol. Vis. Sci. 35, 3938 (1994)
J.M. Deeley, T.W. Mitchell, X. Wei, J. Korth, J.R. Nealon, S.J. Blanksby, R.J.W. Truscott, Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1781, 288 (2008)
M. Rujoi, R. Estrada, M.C. Yappert, Anal. Chem. 76, 1657 (2004)
M. Raguz, J. Widomska, J. Dillon, E.R. Gaillard, W.K. Subczynski, Biochim. Biophys. Acta Biomembr. 1788, 2380 (2009)
M. Rujoi, J. Jin, D. Borchman, D. Tang, M.C. Yappert, Investig. Ophthalmol. Vis. Sci. 44, 1634 (2003)
P.S. Zelenka, Curr. Eye Res. 3, 1337 (1984)
N. Stein, L. Mainali, J.S. Hyde, W.K. Subczynski, Appl. Magn. Reson. 50, 903 (2019)
D.C. Johnston, Phys. Rev. B Condens. Matter Mater. Phys. 74, 184430 (2006)
L. Mainali, M. Raguz, W.J. O’Brien, W.K. Subczynski, Exp. Eye Res. 97, 117 (2012)
R. Estrada, M.C. Yappert, J. Mass Spectrom. 39, 1531 (2004)
R.J. Cenedella, C.R. Fleschner, Curr. Eye Res. 11, 801 (1992)
G. Chandrasekher, R.J. Cenedella, Exp. Eye Res. 60, 707 (1995)
J. Lim, Y.C. Lam, J. Kistler, P.J. Donaldson, Investig. Ophthalmol. Vis. Sci. 46, 2869 (2005)
H. Bloemendal, A. Zweers, F. Vermorken, I. Dunia, E.L. Benedetti, Cell Differ. 1, 91 (1972)
J. Folch, M. Lees, G.H. Sloane Stanley, J. Biol. Chem. 226, 497 (1957)
W.K. Subczynski, C.C. Felix, C.S. Klug, J.S. Hyde, J. Magn. Reson. 176, 244 (2005)
J.T. Buboltz, Rev. Sci. Instrum. 80, 124301 (2009)
J. Huang, J.T. Buboltz, G.W. Feigenson, Biochim. Biophys. Acta Biomembr. 1417, 89 (1999)
L. Mainali, M. Raguz, W.J. O’Brien, W.K. Subczynski, Biochim. Biophys. Acta Biomembr. 1828, 1432 (2013)
L. Mainali, T.G. Camenisch, J.S. Hyde, W.K. Subczynski, Appl. Magn. Reson. 48, 1355 (2017)
F.A. Heberle, G.W. Feigenson, Cold Spring Harb. Perspect. Biol. 3, 1 (2011)
K. Simons, W.L.C. Vaz, Annu. Rev. Biophys. Biomol. Struct. 33, 269 (2004)
S. Arumugam, E.P. Petrov, P. Schwille, Biophys. J. 108, 1104 (2015)
V. Corradi, B.I. Sejdiu, H. Mesa-Galloso, H. Abdizadeh, S.Y. Noskov, S.J. Marrink, D.P. Tieleman, Chem. Rev. 119, 5775 (2019)
T. Fujimoto, I. Parmryd, Front. Cell Dev. Biol. 4, 155 (2017)
D.B. Thibault, C.J. Gillam, A.C. Grey, J. Han, K.L. Schey, J. Am. Soc. Mass Spectrom. 19, 814 (2008)
R.A. Strangeway, J.S. Hyde, T.G. Camenisch, J.W. Sidabras, R.R. Mett, J.R. Anderson, J.J. Ratke, W.K. Subczynski, Cell Biochem. Biophys. 75, 259 (2017)
L. Mainali, J.W. Sidabras, T.G. Camenisch, J.J. Ratke, M. Raguz, J.S. Hyde, W.K. Subczynski, Appl. Magn. Reson. 45, 1343 (2014)
R.R. Mett, J.W. Sidabras, J.R. Anderson, C.S. Klug, J.S. Hyde, J. Magn. Reson. 307, 106585 (2019)
Acknowledgements
Research reported in this publication was supported by the National Eye Institute of the National Institutes of Health under award number R01 EY015526. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Professor James S. Hyde for all the helpful discussions.
Author information
Authors and Affiliations
Contributions
Conceptualization, NS, WKS; performed research, NS; data analysis, NS; writing–original draft preparation, NS; review and editing, WKS; visualization, NS; project administration, WKS; funding acquisition, WKS.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stein, N., Subczynski, W.K. Oxygen Transport Parameter in Plasma Membrane of Eye Lens Fiber Cells by Saturation Recovery EPR. Appl Magn Reson 52, 61–80 (2021). https://doi.org/10.1007/s00723-020-01237-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-020-01237-7