Abstract
Purpose
To investigate the potential etiologies of premature ovarian insufficiency (POI) and diminished ovarian reserve (DOR).
Methods
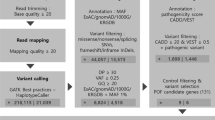
Fourteen women with sporadic POI and 6 women with DOR were enrolled. We used whole-exome sequencing (WES) and bioinformatics analysis to identify variants in a subset of 599 selected POI candidate genes. The identified genes were subjected to gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment and protein-protein interaction (PPI) network analyses to uncover key genes and pathways.
Results
Among the 20 patients, 79 heterozygous variants were detected in 49 genes, which were classified as “likely pathogenic” or “variants of uncertain significance” according to the guidelines of the American College of Medical Genetics and Genomics. Most patients (17/20) carried two or more variants. Monoacylglycerol O-acyltransferase 1 mutations were found in six patients, and cytochrome P450 family 26 subfamily B member 1 and Bardet-Biedl syndrome 9 mutations were each found in four patients. Some variants were shared between DOR and POI. Enrichment analyses showed that the identified genes participate in key ovarian processes, such as follicular development, gonadal development, meiosis, Fanconi anemia, homologous recombination, and transforming growth factor β signaling. A PPI network revealed interactions between these proteins.
Conclusion
Premature ovarian function decline may be polygenic, and overlap exists between the genetic backgrounds of DOR and POI. WES and in silico analyses may be a useful clinical tool for etiological diagnosis and risk prediction for high-risk women in the future.



Similar content being viewed by others
References
Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360(6):606–14. https://doi.org/10.1056/NEJMcp0808697.
Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926–37. https://doi.org/10.1093/humrep/dew027.
Golezar S, Ramezani Tehrani F, Khazaei S, Ebadi A, Keshavarz Z. The global prevalence of primary ovarian insufficiency and early menopause: a meta-analysis. Climacteric. 2019;22(4):403–11. https://doi.org/10.1080/13697137.2019.1574738.
Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol. 2008;68(4):499–509.
Cohen J, Chabbert-Buffet N, Darai E. Diminished ovarian reserve, premature ovarian failure, poor ovarian responder--a plea for universal definitions. J Assist Reprod Genet. 2015;32(12):1709–12. https://doi.org/10.1007/s10815-015-0595-y.
Medicine ASR. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2015;103(3):e9–e17. https://doi.org/10.1016/j.fertnstert.2014.12.093.
Jiao X, Ke H, Qin Y, Chen ZJ. Molecular genetics of premature ovarian insufficiency. Trends Endocrinol Metab. 2018;29(11):795–807. https://doi.org/10.1016/j.tem.2018.07.002.
Franca MM, Mendonca BB. Genetics of primary ovarian insufficiency in the next-generation sequencing era. J Endocr Soc. 2020;4(2):bvz037. https://doi.org/10.1210/jendso/bvz037.
Qin Y, Jiao X, Simpson J, Chen Z. Genetics of primary ovarian insufficiency: new developments and opportunities. Hum Reprod Update. 2015;21(6):787–808.
Man L, Lekovich J, Rosenwaks Z, Gerhardt J. Fragile X-associated diminished ovarian reserve and primary ovarian insufficiency from molecular mechanisms to clinical manifestations. Front Mol Neurosci. 2017;10:290. https://doi.org/10.3389/fnmol.2017.00290.
Tucker EJ, Grover SR, Bachelot A, Touraine P, Sinclair AH. Premature ovarian insufficiency: new perspectives on genetic cause and phenotypic Spectrum. Endocr Rev. 2016;37(6):609–35. https://doi.org/10.1210/er.2016-1047.
Committee opinion no. 605: primary ovarian insufficiency in adolescents and young women. Obstet Gynecol 2014;124(1):193–197. doi:https://doi.org/10.1097/01.AOG.0000451757.51964.98.
Laissue P. The molecular complexity of primary ovarian insufficiency aetiology and the use of massively parallel sequencing. Mol Cell Endocrinol. 2018;460:170–80. https://doi.org/10.1016/j.mce.2017.07.021.
Fonseca DJ, Patino LC, Suarez YC, de Jesus Rodriguez A, Mateus HE, Jimenez KM et al. Next generation sequencing in women affected by nonsyndromic premature ovarian failure displays new potential causative genes and mutations. Fertil Steril. 2015;104(1):154–62.e2. doi:https://doi.org/10.1016/j.fertnstert.2015.04.016.
Bouilly J, Beau I, Barraud S, Bernard V, Azibi K, Fagart J, et al. Identification of multiple gene mutations accounts for a new genetic architecture of primary ovarian insufficiency. J Clin Endocrinol Metab. 2016;101(12):4541–50. https://doi.org/10.1210/jc.2016-2152.
Patino LC, Beau I, Carlosama C, Buitrago JC, Gonzalez R, Suarez CF, et al. New mutations in non-syndromic primary ovarian insufficiency patients identified via whole-exome sequencing. Hum Reprod. 2017;32(7):1512–20. https://doi.org/10.1093/humrep/dex089.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24. https://doi.org/10.1038/gim.2015.30.
Dennis G, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4(5):P3.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. https://doi.org/10.1093/nar/28.1.27.
Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics. 2012;16(5):284–7. https://doi.org/10.1089/omi.2011.0118.
Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45(D1):D362–D8. https://doi.org/10.1093/nar/gkw937.
Rossetti R, Ferrari I, Bonomi M, Persani L. Genetics of primary ovarian insufficiency. Clin Genet. 2017;91(2):183–98. https://doi.org/10.1111/cge.12921.
Xu X, Zhang Y, Zhao S, Bian Y, Ning Y, Qin Y. Mutational analysis of theFAM175A gene in patients with premature ovarian insufficiency. Reprod BioMed Online. 2019;38(6):943–50.
Zhao M, Feng F, Chu C, Yue W, Li L. A novel EIF4ENIF1 mutation associated with a diminished ovarian reserve and premature ovarian insufficiency identified by whole-exome sequencing. J Ovarian Res. 2019;12(1):119. https://doi.org/10.1186/s13048-019-0595-0.
Qin Y, Zhao H, Kovanci E, Simpson JL, Chen ZJ, Rajkovic A. Mutation analysis of NANOS3 in 80 Chinese and 88 Caucasian women with premature ovarian failure. Fertil Steril. 2007;88(5):1465–7. https://doi.org/10.1016/j.fertnstert.2007.01.020.
Zhao S, Li G, Dalgleish R, Vujovic S, Jiao X, Li J, et al. Transcription factor SOHLH1 potentially associated with primary ovarian insufficiency. Fertil Steril. 2015;103(2):548–53.e5. https://doi.org/10.1016/j.fertnstert.2014.11.011.
Qin Y, Jiao X, Dalgleish R, Vujovic S, Li J, Simpson JL, et al. Novel variants in the SOHLH2 gene are implicated in human premature ovarian failure. Fertil Steril. 2014;101(4):1104–9.e6. https://doi.org/10.1016/j.fertnstert.2014.01.001.
Tosh D, Rani HS, Murty US, Deenadayal A, Grover P. Mutational analysis of the FIGLA gene in women with idiopathic premature ovarian failure. Menopause. 2015;22(5):520–6. https://doi.org/10.1097/gme.0000000000000340.
Li L, Wang B, Zhang W, Chen B, Luo M, Wang J, et al. A homozygous NOBOX truncating variant causes defective transcriptional activation and leads to primary ovarian insufficiency. Hum Reprod. 2017;32(1):248–55. https://doi.org/10.1093/humrep/dew271.
Philibert P, Paris F, Lakhal B, Audran F, Gaspari L, Saad A, et al. NR5A1 (SF-1) gene variants in a group of 26 young women with XX primary ovarian insufficiency. Fertil Steril. 2013;99(2):484–9. https://doi.org/10.1016/j.fertnstert.2012.10.026.
Settas N, Anapliotou M, Kanavakis E, Fryssira H, Sofocleous C, Dacou-Voutetakis C, et al. A novel FOXL2 gene mutation and BMP15 variants in a woman with primary ovarian insufficiency and blepharophimosis-ptosis-epicanthus inversus syndrome. Menopause. 2015;22(11):1264–8. https://doi.org/10.1097/gme.0000000000000473.
Laissue P. Aetiological coding sequence variants in non-syndromic premature ovarian failure: from genetic linkage analysis to next generation sequencing. Mol Cell Endocrinol. 2015;411:243–57. https://doi.org/10.1016/j.mce.2015.05.005.
He WB, Banerjee S, Meng LL, Du J, Gong F, Huang H, et al. Whole-exome sequencing identifies a homozygous donor splice-site mutation in STAG3 that causes primary ovarian insufficiency. Clin Genet. 2018;93(2):340–4. https://doi.org/10.1111/cge.13034.
de Vries L, Behar DM, Smirin-Yosef P, Lagovsky I, Tzur S, Basel-Vanagaite L. Exome sequencing reveals SYCE1 mutation associated with autosomal recessive primary ovarian insufficiency. J Clin Endocrinol Metab. 2014;99(10):E2129–32. https://doi.org/10.1210/jc.2014-1268.
Carlosama C, Elzaiat M, Patino LC, Mateus HE, Veitia RA, Laissue P. A homozygous donor splice-site mutation in the meiotic gene MSH4 causes primary ovarian insufficiency. Hum Mol Genet. 2017;26(16):3161–6. https://doi.org/10.1093/hmg/ddx199.
Guo T, Zhao S, Zhao S, Chen M, Li G, Jiao X, et al. Mutations in MSH5 in primary ovarian insufficiency. Hum Mol Genet. 2017;26(8):1452–7. https://doi.org/10.1093/hmg/ddx044.
Wang J, Zhang W, Jiang H, Wu BL. Mutations in HFM1 in recessive primary ovarian insufficiency. N Engl J Med. 2014;370(10):972–4. https://doi.org/10.1056/NEJMc1310150.
Qin Y, Guo T, Li G, Tang TS, Zhao S, Jiao X, et al. CSB-PGBD3 mutations cause premature ovarian failure. PLoS Genet. 2015;11(7):e1005419. https://doi.org/10.1371/journal.pgen.1005419.
Yilmaz NK, Karagin PH, Terzi YK, Kahyaoglu I, Yilmaz S, Erkaya S, et al. BRCA1 and BRCA2 sequence variations detected with next-generation sequencing in patients with premature ovarian insufficiency. J Turk Ger Gynecol Assoc. 2016;17(2):77–82. https://doi.org/10.5152/jtgga.2016.16035.
Weinberg-Shukron A, Rachmiel M, Renbaum P, Gulsuner S, Walsh T, Lobel O, et al. Essential role of BRCA2 in ovarian development and function. N Engl J Med. 2018;379(11):1042–9. https://doi.org/10.1056/NEJMoa1800024.
Desai S, Wood-Trageser M, Matic J, Chipkin J, Jiang H, Bachelot A, et al. MCM8 and MCM9 nucleotide variants in women with primary ovarian insufficiency. J Clin Endocrinol Metab. 2017;102(2):576–82. https://doi.org/10.1210/jc.2016-2565.
Renault L, Patino LC, Magnin F, Delemer B, Young J, Laissue P, et al. BMPR1A and BMPR1B missense mutations cause primary ovarian insufficiency. J Clin Endocrinol Metab. 2019. https://doi.org/10.1210/clinem/dgz226.
Alvaro Mercadal B, Imbert R, Demeestere I, Gervy C, De Leener A, Englert Y, et al. AMH mutations with reduced in vitro bioactivity are related to premature ovarian insufficiency. Hum Reprod. 2015;30(5):1196–202. https://doi.org/10.1093/humrep/dev042.
Liu H, Xu X, Han T, Yan L, Cheng L, Qin Y, et al. A novel homozygous mutation in the FSHR gene is causative for primary ovarian insufficiency. Fertil Steril. 2017;108(6):1050–5.e2. https://doi.org/10.1016/j.fertnstert.2017.09.010.
Gleicher N, Weghofer A, Barad DH. Ovarian reserve determinations suggest new function of FMR1 (fragile X gene) in regulating ovarian ageing. Reprod BioMed Online. 2010;20(6):768–75. https://doi.org/10.1016/j.rbmo.2010.02.020.
Ghezelayagh Z, Totonchi M, Zarei-Moradi S, Asadpour O, Maroufizadeh S, Eftekhari-Yazdi P, et al. The impact of genetic variation and gene expression level of the follicle-stimulating hormone eceptor on ovarian reserve. Cell J. 2018;19(4):620–6. https://doi.org/10.22074/cellj.2018.4183.
Wang TT, Ke ZH, Song Y, Chen LT, Chen XJ, Feng C, et al. Identification of a mutation in GDF9 as a novel cause of diminished ovarian reserve in young women. Hum Reprod. 2013;28(9):2473–81. https://doi.org/10.1093/humrep/det291.
Warman DM, Costanzo M, Marino R, Berensztein E, Galeano J, Ramirez PC, et al. Three new SF-1 (NR5A1) gene mutations in two unrelated families with multiple affected members: within-family variability in 46,XY subjects and low ovarian reserve in fertile 46,XX subjects. Horm Res Paediatr. 2011;75(1):70–7. https://doi.org/10.1159/000320029.
Lin W, Titus S, Moy F, Ginsburg ES, Oktay K. Ovarian aging in women with BRCA germline mutations. J Clin Endocrinol Metab. 2017;102(10):3839–47. https://doi.org/10.1210/jc.2017-00765.
Skiadas CC, Duan S, Correll M, Rubio R, Karaca N, Ginsburg ES, et al. Ovarian reserve status in young women is associated with altered gene expression in membrana granulosa cells. Mol Hum Reprod. 2012;18(7):362–71. https://doi.org/10.1093/molehr/gas008.
Greenseid K, Jindal S, Hurwitz J, Santoro N, Pal L. Differential granulosa cell gene expression in young women with diminished ovarian reserve. Reprod Sci. 2011;18(9):892–9. https://doi.org/10.1177/1933719111398502.
Jindal S, Greenseid K, Berger D, Santoro N, Pal L. Impaired gremlin 1 (GREM1) expression in cumulus cells in young women with diminished ovarian reserve (DOR). J Assist Reprod Genet. 2012;29(2):159–62. https://doi.org/10.1007/s10815-011-9684-8.
Jasti S, Warren BD, McGinnis LK, Kinsey WH, Petroff BK, Petroff MG. The autoimmune regulator prevents premature reproductive senescence in female mice. Biol Reprod. 2012;86(4):110. https://doi.org/10.1095/biolreprod.111.097501.
Tran S, Zhou X, Lafleur C, Calderon MJ, Ellsworth BS, Kimmins S, et al. Impaired fertility and FSH synthesis in gonadotrope-specific Foxl2 knockout mice. Mol Endocrinol. 2013;27(3):407–21. https://doi.org/10.1210/me.2012-1286.
Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383(6600):531–5.
Boyer A, Lapointe E, Zheng X, Cowan RG, Li H, Quirk SM, et al. WNT4 is required for normal ovarian follicle development and female fertility. FASEB J. 2010;24(8):3010–25. https://doi.org/10.1096/fj.09-145789.
Ledent C, Demeestere I, Blum D, Petermans J, Hämäläinen T, Smits G, et al. Premature ovarian aging in mice deficient for Gpr3. Proc Natl Acad Sci U S A. 2005;102(25):8922–6.
McMahon HE, Hashimoto O, Mellon PL, Shimasaki S. Oocyte-specific overexpression of mouse bone morphogenetic protein-15 leads to accelerated folliculogenesis and an early onset of acyclicity in transgenic mice. Endocrinology. 2008;149(6):2807–15. https://doi.org/10.1210/en.2007-1550.
Lyttle Schumacher BM, Jukic AMZ, Steiner AZ. Antimullerian hormone as a risk factor for miscarriage in naturally conceived pregnancies. Fertil Steril. 2018;109(6):1065–71.e1. https://doi.org/10.1016/j.fertnstert.2018.01.039.
Dou X, Guo T, Li G, Zhou L, Qin Y, Chen ZJ. Minichromosome maintenance complex component 8 mutations cause primary ovarian insufficiency. Fertil Steril. 2016;106(6):1485–9.e2. https://doi.org/10.1016/j.fertnstert.2016.08.018.
Ranjha L, Howard SM, Cejka P. Main steps in DNA double-strand break repair: an introduction to homologous recombination and related processes. Chromosoma. 2018;127(2):187–214. https://doi.org/10.1007/s00412-017-0658-1.
Ceccaldi R, Sarangi P, D’Andrea AD. The Fanconi anaemia pathway: new players and new functions. Nat Rev Mol Cell Biol. 2016;17(6):337–49. https://doi.org/10.1038/nrm.2016.48.
Zhang T, Wallis M, Petrovic V, Challis J, Kalitsis P, Hudson DF. Loss of TOP3B leads to increased R-loop formation and genome instability. Open Biol. 2019;9(12):190222. https://doi.org/10.1098/rsob.190222.
Kwan KY, Moens PB, Wang JC. Infertility and aneuploidy in mice lacking a type IA DNA topoisomerase III. beta Proc Natl Acad Sci U S A. 2003;100(5):2526–31.
Räschle M, Knipscheer P, Knipsheer P, Enoiu M, Angelov T, Sun J, et al. Mechanism of replication-coupled DNA interstrand crosslink repair. Cell. 2008;134(6):969–80. https://doi.org/10.1016/j.cell.2008.08.030.
Yang X, Zhang X, Jiao J, Zhang F, Pan Y, Wang Q, et al. Rare variants in FANCA induce premature ovarian insufficiency. Hum Genet. 2019;138(11–12):1227–36. https://doi.org/10.1007/s00439-019-02059-9.
Yang Y, Guo T, Liu R, Ke H, Xu W, Zhao S, et al. FANCL gene mutations in premature ovarian insufficiency. Hum Mutat. 2020;41(5):1033–41. https://doi.org/10.1002/humu.23997.
Pastore L, Manichaikul A, Wang X, Finkelstein J. FMR1 CGG repeats: reference levels and race-ethnic variation in women with normal fertility (study of women’s health across the nation). Reprod Sci. 2016;23(9):1225–33.
Acknowledgments
The authors are deeply grateful to all participants involved in this study and all the doctors and researchers who participated in the study.
Funding
This study was supported by the National Key Research and Development Program [grant number 2018YFC1002105]; CAMS Innovation Fund for Medical Sciences (CIFMS) [grant number 2017-I2M-1-002].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, material preparation, data collection, and analysis. The first draft of the manuscript was written by R. T, and Q. Y revised it. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
This study was approved by the Ethics Committee of our hospital.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee of Peking Union Medical College Hospital and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 68 kb)
Rights and permissions
About this article
Cite this article
Tang, R., Yu, Q. Novel variants in women with premature ovarian function decline identified via whole-exome sequencing. J Assist Reprod Genet 37, 2487–2502 (2020). https://doi.org/10.1007/s10815-020-01919-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01919-y