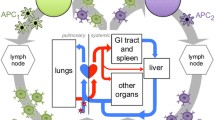
Abstract
Metastatic seeding of distant organs can occur in the very early stages of primary tumor development. Once seeded, these micrometastases may enter a dormant phase that can last decades. Curiously, the surgical removal of the primary tumor can stimulate the accelerated growth of distant metastases, a phenomenon known as metastatic blow-up. Recent clinical evidence has shown that the immune response can have strong tumor promoting effects. In this work, we investigate if the pro-tumor effects of the immune response can have a significant contribution to metastatic dormancy and metastatic blow-up. We develop an ordinary differential equation model of the immune-mediated theory of metastasis. We include both anti- and pro-tumor immune effects, in addition to the experimentally observed phenomenon of tumor-induced immune cell phenotypic plasticity. Using geometric singular perturbation analysis, we derive a rather simple model that captures the main processes and, at the same time, can be fully analyzed. Literature-derived parameter estimates are obtained, and model robustness is demonstrated through a time dependent sensitivity analysis. We determine conditions under which the parameterized model can successfully explain both metastatic dormancy and blow-up. The results confirm the significant active role of the immune system in the metastatic process. Numerical simulations suggest a novel measure to predict the occurrence of future metastatic blow-up in addition to new potential avenues for treatment of clinically undetectable micrometastases.












Similar content being viewed by others
References
Aguirre-Chiso J (2007) Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer 7(11):834–846
Avanzini S, Antal T (2019) Cancer recurrence times from a branching process model. PLoS Comput Biol 15(11):e1007423
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357:539–545
Benzekry S, Gandolfi A, Hahnfeldt P (2014) Global dormancy of metastases due to systemic inhibition of angiogenesis. PLoS ONE 9(1):e84249
Benzekry S, Lamont C, Barbolosi D et al (2017) Mathematical modeling of tumor-tumor distant interactions supports a systemic control of tumor growth. Cancer Res 77(18):5183–5193
Benzekry S, Sentis C, Coze C, Tessonnier L, Andre N (2020) Descriptive and prognostic value of a computational model of metastasis in high-risk neuroblastoma. MedRxiv. https://doi.org/10.1101/2020.03.26.20042192
Blomberg O, Spagnuolo L, de Visser K (2018) Immune regulation of metastasis: mechanistic insights and therapeutic opportunities. Dis Model Mech. https://doi.org/10.1242/dmm.036236
Cameron M et al (2000) Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res 60(9):2541–2546
Chambers A, Groom A, MacDonald I (2002) Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2(8):563–572
Chiarella P, Bruzzo J, Meiss R, Ruggiero R (2012) Concomitant tumor resistance. Cancer Lett 324(2):133–141
Cohen E, Gao H, Anfossi S et al (2015) Inflammation mediated metastasis: immune induced epithelial-to-mesenchymal transition in inflammatory breast cancer cells. PLoS One 10(7):e0132710
Colegio O, Chu N, Szabo A et al (2014) Functional polarization of tumor-associated macrophages by tumour-derived lactic acid. Nature 513:559–563
Coughlin C, Murray J (2010) Current and emerging concepts in tumour metastasis. J Pathol. https://doi.org/10.1002/path.2727
de Mingo Pulido A, Ruffell B (2016) Immune regulation of the metastatic process: implications for therapy. Adv Cancer Res 132:139–163
Del Monte U (2009) Does the cell number \(10^{9}\) still really fit one gram of tumor tissue? Cell Cycle 8(3):505–506
den Breems N, Eftimie R (2016) The re-polarisation of M2 and M1 macrophages and its role on cancer outomes. J Theor Biol 390:23–39. https://doi.org/10.1016/j.jtbi.2015.10.063
Dewan M, Galloway A, Kawashima N et al (2009) Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-ctla-4 antibody. Clin Cancer Res 15(17):5379–5388. https://doi.org/10.1158/1078-0432.CCR-09-0265
Eftimie R, Bramson J, Earn D (2011) Interactions between the immune system and cancer: a brief review of non-spatial mathematical models. Bull Math Biol 73(1):2–32
Eikenberry S, Thalhauser C, Kuang Y (2009) Tumor-immune interaction, surgical treatment, and cancer recurrence in a mathematical model of melanoma. PLoS Comput Biol 5(4):e1000362. https://doi.org/10.1371/journal.pcbi.1000362
El-Kenawi A, Gatenby C, Robertson-Tessi M et al (2019) Acidity promotes tumour progression by altering macrophage phenotype in prostate cancer. Brit J Cancer 121:556–566. https://doi.org/10.1038/s41416-019-0542-2
Emens L, Ascierto P, Darcy P et al (2017) Cancer immunotherapy: opportunities and challenges in the rapidly evolving clinical landscape. Eur J Cancer 81:116–129
Forget P, Vandengende J, Berliere M et al (2010) Do intraoperative analgesics influence breast cancer recurrence after mastectomy? a retrospective analysis. Anesth Analg 110(6):1630–1635
Franßen L, Chaplain M (2019) A mathematical multi-organ model for bidirectional epithelial-mesenchymal transitions in the metastatic spread of cancer. BioRxiv. https://doi.org/10.1101/745547
Franßen L, Lorenzi T, Burgess A et al (2019) A mathematical framework for modelling the metastatic spread of cancer. Bull Math Biol 81:1965–2010
Frei C, Hillen T, Rhodes A (2020) A stochastic model for cancer metastasis: branching stochastic process with settlement. Math Med Biol 37(2):153–182
Friberg S, Nystrom A (2015) Cancer metastases: early dissemination and late recurrences. Cancer Growth Metastasis 8:43–49
Giancotti F (2013) Mechanisms governing metastatic dormancy and reactivation. Cell 155(4):750–764
Goddard E, Bozic I, Riddell S, Ghajar C (2018) Dormant tumour cells, their niches and the influence of immunity. Nat Cell Biol 20(11):1240–1249
Gorelik E (1983) Concomitant tumor immunity and the resistance to a second tumor challenge. Adv Cancer Res 39:75–120
Griffiths J, Wallet P, Pflieger L, Stenehjem D et al (2020) Circulating immune cell phenotype dynamics reflect the strength of tumor-immune interactions in patients during immunotherapy. Proc Natl Acad Sci USA 117(27):16072–16082
Hanin L, Rose J (2018) Suppression of metastasis by primary tumor and acceleration of metastasis following primary tumor resection: A natural law? B Math Biol 80(3):519–539
Hanin L, Seidel K, Stoevesandt D (2016) A universal model of metastatic cancer, its parametric forms and their identification: what can be learned from site-specific volumes of metastases. J Math Biol 72:1633–1662. https://doi.org/10.1007/s00285-015-0928-6
Hek G (2010) Geometric singular perturbation theory in biological practice. J Math Biol 60(3):347–386
Hobson J, Gummadidala P, Silverstrim B et al (2013) Acute inflammation induced by the biopsy of mouse mammary tumors promotes the development of metastasis. Breast Cancer Res Tr 139(2):391–401
Jones C (1995) Geometric singular perturbation theory. In: Russell J (ed) Dynamical systems, 2nd session of the centro internazionale matematico estivo (CIME). Springer, Berlin, pp 44–118
Joyce J, Pollard J (2009) Microenvironmental regulation of metastasis. Nat Rev Cancer 9(4):239–252. https://doi.org/10.1038/nrc2618
Kaplan R, Riba R, Zacharoulis S et al (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438:820–827. https://doi.org/10.1038/nature04186
Kim Y, Jeon H, Othmer H (2017) The role of the tumor microenvironment in glioblastoma: a mathematical model. IEEE Trans Biomed Eng 64(3):519–527
Kumar G, Manjunatha B (2013) Metastatic tumors to the jaws and oral cavity. J Oral Maxillofac Pathol 17(1):71–75
Kuznetsov V, Makalkin V, Taylor I et al (1994) Nonlinear dynamics of immunogenic tumors: parameter estimation and global bifurcation analysis. Bull Math Biol 50(2):295–321
Lai X, Friedman A (2019) How to schedule VEGF and PD-1 inhibitors in combination cancer therapy? BMC Syst Biol 13(1):30
Liao K, Bai X, Friedman A (2013) The role of CD200-CD200R in tumor immune evasion. J Theor Biol 328:65–76
Liao K, Bai X, Friedman A (2014) A mathematical modeling of interleukin-35 promoting tumor growth and angiogenesis. PLoS One 9(10):e110126
Liu V, Wong L, Jang T et al (2007) Tumor evasion of the immune system by converting CD4+CD25- T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-\(\beta \). J Immunol 178:2883–2892
Liu Y, Cao X (2016) Immunosuppressive cells in tumor immune escape and metastasis. J Mol Med 94(5):509–522. https://doi.org/10.1007/s00109-015-1376-x
Luzzi K et al (1998) Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol. https://doi.org/10.1016/S0002-9440(10)65628-3
Maida V, Ennis M, Kuziemsky C, Corban J (2009) Wounds and survival in cancer patients. Eur J Cancer 45(18):3237–3244
Marx J (2004) Inflammation and cancer: the link grows stronger. Science 306(5698):966–968
Michelson S, Leith J (1994) Dormancy, regression and recurrence: towards a unifying theory of tumor growth control. J Theor Biol 169:327–338
Michelson S, Leith J (1995) A theoretical explanation of concomitant resistance. Bull Math Biol 57(5):733–747
Neophytou C, Kyriakou T, Papageorgis P (2019) Mechanisms of metastatic tumor dormancy and implications for cancer therapy. Int J Mol Sci 20(24):6158
Norton K, Jin K, Popel AS (2018) Modeling triple-negative breast cancer heterogeneity: effects of stromal macrophages, fibroblasts and tumor vasculature. J Theor Biol 452:56–68
Oleinika K, Nibbs R, Graham G et al (2013) Suppression, subversion and escape: the role of regulatory t cells in cancer progression. Clin Exp Immunol 171(1):36–45
Olobatuyi O, de Vries G, Hillen T (2017) A reaction-diffusion model for radiation-induced bystander effects. J Math Biol 75(2):341–372
Park C, Hartl C, Schmid D et al (2018) Extended release of perioperative immunotherapy prevents tumor recurrence and eliminates metastases. Sci Transl Med 10(433):eaar1916
Pascual M, Alonso S, Pares D et al (2010) Randomized clinical trial comparing inflammatory and angiogenic response after open versus laparoscopic curative resection for colonic cancer. Brit J Surg 98:50–59
Poleszczuk J, Luddy K, Prokopiou S et al (2016) Abscopal benefits of localized radiotherapy depend on activated T-cell trafficking and distribution between metastatic lesions. Cancer Res 76(5):1009–1018. https://doi.org/10.1158/0008-5472.CAN-15-1423
Prehn R (1993) Two competing influences that may explain concomitant tumor resistance. Cancer Res 53(14):3266–3269
Retsky M, Demicheli R, Hrushesky W et al (2013) Reduction of breast cancer relapses with perioperative non-steroidal anti-inflammatory drugs: new findings and a review. Curr Med Chem 20(33):4163–4176
Rhodes A, Hillen T (2019) A mathematical model of the immune-mediated theory of metastasis. J Theor Biol 482:109999
Riggi N, Auget M, Stamenkovic I (2018) Cancer metastasis: a reappraisal of its underlying mechanisms and their relevance to treatment. Annu Rev Pathol Mech 13:117–140
Robertson-Tessi M, El-Kareh A, Goriely A (2012) A mathematical model of tumor-immune interactions. J Theor Biol 294:56–73
Ruggiero R, Bruzzo J, Chiarella P, Bustuoabad O et al (2012) Concomitant tumor resistance: the role of tyrosine isomers in the mechanisms of metastases control. Cancer Res 72(5):1043–1050
Shahriyari L (2016) A new hypothesis: some metastases are the result of inflammatory processes by adapted cells, especially adapted immune cells at sites of inflammation. F1000Research. https://doi.org/10.12388/f1000research.8055.1
Tyzzer E (1913) Factors in the production and growth of tumor metastases. J Med Res 28(2):309–332
Valastyan S, Weinberg R (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147(2):275–292. https://doi.org/10.1016/j.cell.2011.09.024
Walker R, Poleszczuk J, Pilon-Thomas S et al (2018) Immune interconnectivity of anatomically distant tumors as a potential mediator of systemic responses to local therapy. Sci Rep 8(1):1–11
Walker R, Schoenfeld J, Pilon-Thomas S et al (2017) Evaluating the potential for maximized T cell redistribution entropy to improve abscopal responses to radiotherapy. Converg Sci Phys Oncol 3:034001
Walter N, Rice P, Redente E et al (2011) Wound healing after trauma may predispose to lung cancer metastasis: Review of potential mechanisms. Am J Resp Cell Mol 44(5):591–596. https://doi.org/10.1165/rcmb.2010-0187RT
Weiss L (1990) Metastatic inefficiency. Adv Cancer Res 54:159–211
Wilkie K (2013) A review of mathematical models of cancer-immune interactions in the context of tumor dormancy. In: Enderling H, Almong N, Hlatky L (eds) Systems biology of tumor dormancy. Springer, Berlin, pp 201–234
Wilkie K, Aktar F (2020) Mathematically modeling inflammation as a promoter of tumour growth. BioRxiv. https://doi.org/10.1101/2020.03.08.982918
Wilkie K, Hahnfeldt P (2017) Modeling the dichotomy of the immune response to cancer: cytotoxic effects and tumor-promoting inflammation. Bull Math Biol 79(6):1426–1448
Yang Z, Tang X, Meng G et al (2019) Latent cytomegalovirus infection in female mice increases breast cancer metastasis. Cancers 11(447):1–20
Acknowledgements
We would like to thank the referees for their helpful detailed comments. AR gratefully acknowledges support from an Alberta Innovates Graduate Student Scholarship. TH is supported by an NSERC Discovery Grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Here we prove Proposition 3 and provide additional details concerning the parameter sensitivity analysis reported in Fig. 4. We begin by proving Proposition 3.
Proof
Consider \((u,x(u), y(u))\in {\mathcal {M}}\). We compute the derivatives \(x_{u}(u)\) and \(y_{u}(u)\). We begin with \(x_{u}\):
The sign of \(x_{u}\) depends entirely on the sign of
With the choices we made for \(\lambda (u)\) and ed(u), we arrive at the following chain of equivalent conditions:
The roots to the above quadratic are given by
This gives distinct, real roots (assuming that \(a_{1}b_{1}\ne 0\)) with at least one of them negative. If \(u_{+} > 0\), then we see that \(x_{u} > 0\) for \(u < u_{+}\) and negative otherwise. If \(u_{+} < 0\), then we simply have \(x_{u} < 0\) for all non-negative u.
Next, we consider \(y_{u}\). We show simply that \(y_{u}\) is non-negative, as
The final inequality results from the fact that \((\psi + ed(u)x(u))f'(u) \ge 0\) since f is increasing (A1). Now, we can use the expressions for x(u) and \(x_{u}\), as well as our choice of \(ed(u) = \chi u\) to arrive at
The sign of this expression depends only on the sign of the term
By assumption (A2), the denominator is always negative, and therefore the sign of the above expression is determined by the sign of its numerator. With the choices for ed(u) and \(\lambda (u)\) from Sect. 2.3, the numerator simplifies to
Using the fact that \(a_{1}-\omega < 0\) (A2) guarantees that the quadratic is negative for all \(u \ge 0\), and therefore \(y_{u}(u) \ge 0\) for all \(u \ge 0\). \(\square \)
Rights and permissions
About this article
Cite this article
Rhodes, A., Hillen, T. Implications of immune-mediated metastatic growth on metastatic dormancy, blow-up, early detection, and treatment. J. Math. Biol. 81, 799–843 (2020). https://doi.org/10.1007/s00285-020-01521-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-020-01521-x
Keywords
- Mathematical oncology
- Cancer modeling
- Metastasis
- Dormancy
- Geometric singular perturbation analysis
- Ordinary differential equations