Abstract
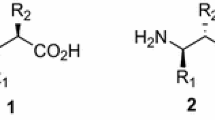
A 6-step enantioselective synthesis of (2S,3R)-3-alkyl/alkenylglutamates, including the biologically significant amino acid, (2S,3R)-3-methylglutamate, protected for Fmoc SPPS, is reported. Overall yields range from 52–65%. Key to the success of these syntheses was the development of a high-yielding 2-step synthesis of Fmoc Garner’s aldehyde followed by a Horner–Wadsworth–Emmons reaction to give the corresponding Fmoc Garner’s enoate in a 94% yield. The diastereoselective 1,4-addition of lithium dialkylcuprates to the Fmoc Garner’s enoate was explored. Significant decomposition occurred when using lithium diethylcuprate and conditions previously reported for the 1,4-addition of lithium dialkylcuprates to Boc or Cbz-protected Garner’s enoate. An optimization study of this reaction resulted in a robust set of conditions that addressed the shortcomings of previously reported conditions. Under these conditions, highly diastereoselective (> 20:1 in most cases) 1,4-addition reactions of lithium dialkyl/dialkenylcuprates to the Fmoc Garner’s enoate were achieved in 76–99% yield. The resulting 1,4-addition products were easily converted into the Fmoc-(2S,3R)-3-alkyl/alkenylglutamates in two steps.




Similar content being viewed by others
Availability of data and material
NMR spectra for 1, 5, 6, 9–21 and variable temperature 1H NMR experiments for 6, 12–16 are provided in the supplementary information file.
Notes
Cubist Pharmaceuticals Inc. has described in a patent the synthesis of Fmoc-(2S,3R)-3MeGluOallyl in which the key step was the conjugate 1,4-addition of Me2CuLi to the t-butyl ester derivative of oxazolidine 2. Neither the dr of the conjugate addition reaction nor the enantiomeric purity of the Fmoc-3MeGluOallyl was reported.
All the temperatures listed in this paper are internal temperatures that were measured throughout the reaction.
The anti-isomer could not be detected at 60 °C in DMSO-d6.
The reaction was almost quantitative on scales as high as 5 g. On a 10 g scale, the yield dropped to 79% due to the intermediate betaine oiling out which inhibited stirring. In all instances, the reaction proceeds with very high diastereoselectivity and any traces of cis-isomer were easily removed by chromatography.
House and coworkers noted that vinyl cuprates begin to decompose at temperatures > -25 °C (House and Wilkins 1978).
References
Alexander DC, Baltz RH, Brian P, Coeffet-Le Ga M-F, Doekel, S, He X, Kulkarni V, Leitheiser C, Miao VPW, Nguyen K. T, Parr IB, Ritz D, Zhang Y (2006) Antiinfective Lipopeptides. Patent WO 110185A2. See Chem Abstr 145:455271
Bertz SH, Dabbagh G (1984) organocuprate reactions with cyclopropanes. evidence for three types of mechanism. J Org Chem 49:1739–1743
Bertz SH, Dabbagh G, Mujsce AM (1991) Reaction of Bu2CuLi * LiI with alkyl iodides: evidence for free radicals and electron transfer. J Am Chem Soc 113:631–636
Bertz SH, Gibson CP, Dabbagh G (1987) Preparation of lithium organocuprates from various Cu(I) salts. Tetrahedron Lett 37:4251–4254
Buzhe X, Yann H, Sung-Hyun Y, Harris PWR, Brimble MAA (2019) Versatile Boc solid phase synthesis of daptomycin and analogues using site specific, on-resin ozonolysis to install the kynurenine residue. Chem Eur J 25:14101–14107
Chen D, Chow HY, Po KHL, Ma W, Leung ELY, Sun Z, Liu M, Chen S, Li X (2019) Total synthesis and structural establishment/revision of antibiotics A54145. Org Lett 21:5639–5644
Chiou W-H, Schoenfelder A, Sun L, Mann A, Ojima I (2007) Rhodium-catalyzed cyclohydrocarbonylation approach to the syntheses of enantiopure homokainoids. J Org Chem 72:9418–9425
Cong X, Hu F, Liu K-G, Liao Q-J, Yao Z-J (2005) Chemoselective deprotection of cyclic N, O-aminals using catalytic bismuth(III) bromide in acetonitrile. J Org Chem 70:4514–4516
Cowell SM, Lee YS, Cain JP, Hruby VJ (2004) Exploring ramachandran and chi space: conformationally constrained amino acids and peptides in the design of bioactive polypeptide ligands. Curr Med Chem 11:2785–2798
Eliasof S, McIlvain HB, Petroski RE, Foster AC, Dunlop J (2001) Pharmacological characterization of threo-3-methylglutamic acid with excitatory amino acid transporters in native and recombinant systems. J Neurochem 77:550
Eash KJ, Pulia MS, Wieland LC, Mohan RS (2000) A simple chemoselective method for the deprotection of acetals and ketals using bismuth nitrate pentahydrate. J Org Chem 65:8399–8401
Fan S, Liu S, Zhu S, Feng J, Zhang Z, Huang J (2017) Temperature-dependent enantio- and diastereodivergent synthesis of amino acids with one or multiple chiral centers. Org Lett 19:4660–4663
Flamant-Robin C, Wang Q, Chiaroni A, Sasaki NA (2002) An efficient method for the stereoselective synthesis of cis-3-substituted prolines: conformationally constrained α-amino acids. Tetrahedron 58:10475–10484
Han G, Hruby VJ (2001) A study of conjugate addition to a γ, δ-dioxolanyl-α, β-unsaturated ester. Tetrahedron Lett 42:4281–4283
Hanessian S, Sumi K (1991) On the Stereochemical Divergence in the Conjugate Addition of Lithium Dimethylcuprate/Trimethylsilyl Chloride to γ-Alkoxy and γ-Ureido α, β-Unsaturated Esters. Synthesis (Stuttg) 12:1083–1089
Hanessian S, Wang W, Gai Y (1996) Stereocontrolled exploiting internal sequential functionalization in acyclic systems by 1,2-asymmetric induction—generation of symmetry-related. Tetrahedron Lett 37:7477–7480
Hartzoulakis B, Gani D (1994) Syntheses of (2S,3R)- and (2S,3S)-3-methylglutamic acid. J Chem Soc Perkin Trans 1:2525–2531
Herdeis C, Hubmaan P (1994) Synthesis of homochiral 3-substituted glutamic acids and prolines from pyroglutamic acid. Tetrahedron Asymm 5:351–354
Herdeis C, Kelm B (2003) A stereoselective synthesis of 3-substituted (S)-pyroglutamic and glutamic acids via OBO ester derivatives. Tetrahedron 59:217–229
House HO, Chu CY, Wilkins JM, Umen MJ (1975) The Chemistry of carbanions. XXVII. convenient precursor for the generation of lithium organocuprates. J Org Chem 40:1460–1469
House HO, Wilkins JM (1978) Reactions involving electron transfer. 12. effects of solvent and substituents upon the ability of lithium diorganocuprates to add to enones. J Org Chem 43:2443–2454
Hruby VJ, Li G, Haskell-Luevano C, Shenderovich MD (1997) Design of peptides, proteins, and peptidomimetics in chi space. Biopolymers 43:219–266
Jako I, Uiber P, Mann A, Wermuth CG, Boulanger T, Norberg B, Evrard G, Durant F (1991) Stereoselective synthesis of 3-alkylated glutamic acids: application to the synthesis of secokainic acid. J Org Chem 56:5729–5733
Jiang S, Li P, Lai CC, Kelley JA, Roller PP (2006) Design and concise synthesis of fully protected analogues of L-γ-carboxyglutamic acid. J Org Chem 71:7307–7314
Kralt B, Moreira R, Palmer M, Taylor SD (2019) Total synthesis of A54145 factor D. J Org Chem 84:12021–12030
Kumar S, Flamant-Robin C, Wang Q, Chiaroni A, Sasaki NA (2005) Synthesis of 4-substituted-3-aminopiperidin-2-ones: application to the synthesis of a conformationally constrained tetrapeptide N-acetyl-Ser-Asp-Lys-Pro. J Org Chem 70:5946–5953
Lam HY, Zhang Y, Liu H, Xu J, Wong CTT, Xu C, Li X (2013) Total synthesis of daptomycin by cyclization via a chemoselective serine ligation. J Am Chem Soc 135:6272–6279
Lohani CR, Taylor R, Palmer M, Taylor SD (2015) Solid-phase total synthesis of daptomycin and analogs. Org Lett 17:748–751
Lou S, McKenna GM, Tymonko SA, Ramirez A, Benkovics T, Conlon DA, González-Bobes F (2015) Syn-selective synthesis of β-branched α-amino acids by alkylation of Glycine-derived imines with secondary sulfonates. Org Lett 17:5000–5008
Marshall JA, Beaudoin S (1996) Stereoselective synthesis of differentially protected derivatives of the higher amino sugars destomic acid and lincosamine from serine and threonine. J Org Chem 61:581–586
Pattenden G, Ashweek NJ, Baker-Glenn CAG, Kempson J, Walker GM, Yee JGK (2008) Total synthesis of (−)-Ulapualide A, a novel tris-oxazole macrolide from marine nudibranchs, based on some biosynthesis speculation. Org Biomol Chem 6:1478–1497
Pradhan PP, Bobbitt JM, Bailey WF (2009) Oxidative cleavage of benzylic and related ethers, using an oxoammonium salt. J Org Chem 74:9524–9527
Ranatunga S, Tang C-HA, Hu C-CA, Del Valle JR (2012) Total synthesis and structural revision of lucentamycin A. J Org Chem 77:9859–9864
Rush J, Bertozzi CR (2006) An α-formylglycine building block for Fmoc-based solid-phase peptide synthesis. Org Lett 8:131–134
Soloshonok VA, Cai C, Hruby VJ (1999a) Asymmetric michael addition reactions of chiral Ni(II)-complex of glycine with (N-trans-enoyl)oxazolidines: Improved reactivity and stereochemical outcome. Tetrahedron Asymmetry 10:4265–4269
Soloshonok VA, Cai C, Hruby VJ, Van Meervelt L (1999b) Asymmetric synthesis of novel highly stericaily constrained trifluoromethyl-4-methylpyroglutamic acids. Tetrahedron 55:12045–12058
Soloshonok VA, Cai C, Hruby VJ (2000) A practical asymmetric synthesis of enantiomerically pure 3-substituted pyroglutamic acids and related compounds. Angew Chemie Int Ed 39:2172–2175
Soloshonok VA, Izawa K (2009) Asymmetric synthesis and application of α-amino acids. ACS Symposium Series 1009. American Chemical Society. Chicago, USA
Soloshonok VA, Ueki H, Tiwari R et al (2004) Virtually complete control of simple and face diastereoselectivity in the Michael addition reactions between achiral equivalents of a nucleophilic glycine and (S)- or (R)-3-(E-enoyl)-4-phenyl-1,3-oxazolidin-2-ones: practical method for preparation of β-su. J Org Chem 69:4984–4990
Spangenberg T, Schoenfelder A, Breit B, Mann A (2010) 1,2-diastereoselective C-C bond-forming reactions for the synthesis of chiral β-branched α-amino acids. European J Org Chem 2010:6005–6018
Suzuki K, Seebach D (1992) threo-3-Alkyl- and -Arylglutamic Acid Derivatives by Michael Additions of Boc-BMI Li-Enolates to 2,6-Di-t-butyl-4-methoxyphenyl Alkenoates On the Diastereoselectivity of the Coupling of Trigonal Centers Involving Heterocyclic Li-Enolates. Liebigs Ann Chem 1992:51–61
Takano S, Yoshinori S, Ogasawara K (1988) Concurrent reductive cleavage and recombination of γ,δ-AIkylidenedioxy-α,β- unsaturated esters promoted by organocuprates. Chem Commun: 449–450
Tokairin Y, Soloshonok VA, Moriwaki H, Konno H (2019) Asymmetric synthesis of (2S,3S)-3-Me-glutamine and (R)-allo-threonine derivatives proper for solid-phase peptide coupling. Amino Acids 51:419–432
Wood TM, Martin NI (2019) The calcium-dependent lipopeptide antibiotics: structure, mechanism, and medicinal chemistry. Med Chem Comm 10:634–646
Wu Y-D, Gellman S (2008) Peptidomimetics. Acc Chem Res 41:1231–1232
Funding
This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant to S.D.T. (04233–2017). R.M. is grateful to NSERC for a post-graduate scholarship.
Author information
Authors and Affiliations
Contributions
Both authors contributed to the preparation of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Informed consent
No experiments involving living things were preformed so informed consent is not required.
Additional information
Handling editor: V. Soloshonok.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moreira, R., Taylor, S.D. Highly efficient and enantioselective syntheses of (2S,3R)-3-alkyl- and alkenylglutamates from Fmoc-protected Garner’s aldehyde. Amino Acids 52, 987–998 (2020). https://doi.org/10.1007/s00726-020-02868-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-020-02868-7