Abstract
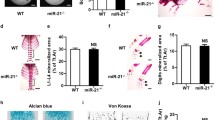
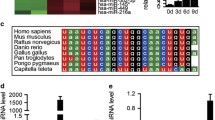
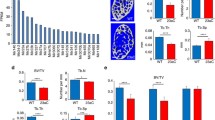
Maternal nutrition is crucial for the offspring’s skeleton development and the onset of osteoporosis later in life. While maternal low protein diet has been shown to regulate bone mass negatively, the effect of a high protein diet (HP) remains unexplored. Here, we found that C57BL/6 mice fed with HP delivered offspring with decreased skeletal mineralization at birth and reduced bone mass throughout their life due to a decline in their osteoblast maturation. A small RNA sequencing study revealed that miR-24-1-5p was highly upregulated in HP group osteoblasts. Target prediction and validation studies identified SMAD-5 as a direct target of miR-24-1-5p. Furthermore, mimic and inhibitor studies showed a negative correlation between miR-24-1-5p expression and osteoblast function. Moreover, ex vivo inhibition of miR-24-1-5p reversed the reduced maturation and SMAD-5 expression in the HP group osteoblasts. Together, we show that maternal HP diminishes the bone mass of the offspring through miR-24-1-5p.








Similar content being viewed by others
References
Cooper C et al (1997) Growth in infancy and bone mass in later life. Ann Rheum Dis 56(1):17–21
Cooper C et al (2002) The fetal origins of osteoporotic fracture. Calcif Tissue Int 70(5):391–394
Jordan KM, Cooper C (2002) Epidemiology of osteoporosis. Best Pract Res Clin Rheumatol 16(5):795–806
Thompson JN (2007) Fetal nutrition and adult hypertension, diabetes, obesity, and coronary artery disease. Neonatal Netw 26(4):235–240
Cleal JK et al (2007) Mismatched pre- and postnatal nutrition leads to cardiovascular dysfunction and altered renal function in adulthood. Proc Natl Acad Sci USA 104(22):9529–9533
Stocker CJ, Arch JR, Cawthorne MA (2005) Fetal origins of insulin resistance and obesity. Proc Nutr Soc 64(2):143–151
Simmons R (2011) Epigenetics and maternal nutrition: nature v. nurture. Proc Nutr Soc 70(1):73–81
Goyal D, Limesand SW, Goyal R (2019) Epigenetic responses and the developmental origins of health and disease. J Endocrinol 242(1):T105–T119
Baird J et al (2011) Does birthweight predict bone mass in adulthood? A systematic review and meta-analysis. Osteoporos Int 22(5):1323–1334
Flanagan DE et al (1999) Reduced foetal growth and growth hormone secretion in adult life. Clin Endocrinol 50(6):735–740
Javaid MK et al (2006) Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet 367(9504):36–43
Zhu K et al (2014) Maternal vitamin D status during pregnancy and bone mass in offspring at 20 years of age: a prospective cohort study. J Bone Miner Res 29(5):1088–1095
Ganpule A et al (2006) Bone mass in Indian children-relationships to maternal nutritional status and diet during pregnancy: the Pune Maternal Nutrition Study. J Clin Endocrinol Metab 91(8):2994–3001
Villa CR et al (2016) Maternal vitamin D beneficially programs metabolic, gut and bone health of mouse male offspring in an obesogenic environment. Int J Obes 40(12):1875–1883
Suntornsaratoon P, Krishnamra N, Charoenphandhu N (2015) Positive long-term outcomes from presuckling calcium supplementation in lactating rats and the offspring. Am J Physiol Endocrinol Metab 308(11):E1010–E1022
Hastings-Roberts MM, Zeman FJ (1977) Effects of protein deficiency, pair-feeding, or diet supplementation on maternal, fetal and placental growth in rats. J Nutr 107(6):973–982
Mehta G et al (2002) Intrauterine exposure to a maternal low protein diet reduces adult bone mass and alters growth plate morphology in rats. Calcif Tissue Int 71(6):493–498
Andreasyan K et al (2007) Higher maternal dietary protein intake in late pregnancy is associated with a lower infant ponderal index at birth. Eur J Clin Nutr 61(4):498–508
Blumfield ML et al (2012) Systematic review and meta-analysis of energy and macronutrient intakes during pregnancy in developed countries. Nutr Rev 70(6):322–336
Rush D, Stein Z, Susser M (1980) A randomized controlled trial of prenatal nutritional supplementation in New York City. Pediatrics 65(4):683–697
Ota E et al (2012) Antenatal dietary advice and supplementation to increase energy and protein intake. Cochrane Database Syst Rev 9:CD000032
Sloan NL et al (2001) The effect of prenatal dietary protein intake on birth weight. Nutr Res 21(1–2):129–139
Rigueur D, Lyons KM (2014) Whole-mount skeletal staining. Methods Mol Biol 1130:113–121
Ali SJ et al (2019) Bone loss in MPTP mouse model of Parkinson’s disease is triggered by decreased osteoblastogenesis and increased osteoclastogenesis. Toxicol Appl Pharmacol 363:154–163
Sharan K et al (2017) Regulation of bone mass through pineal-derived melatonin-MT2 receptor pathway. J Pineal Res 63(2):e12423
Ali SJ et al (2019) Chlorpyrifos exposure induces parkinsonian symptoms and associated bone loss in adult swiss albino mice. Neurotox Res 36(4):700–711
Roman-Garcia P et al (2014) Vitamin B(1)(2)-dependent taurine synthesis regulates growth and bone mass. J Clin Invest 124(7):2988–3002
Lewis KE et al (2017) Skeletal site-specific changes in bone mass in a genetic mouse model for human 15q11-13 duplication seen in Autism. Sci Rep 7(1):9902
Sharan K et al (2011) A novel quercetin analogue from a medicinal plant promotes peak bone mass achievement and bone healing after injury and exerts an anabolic effect on osteoporotic bone: the role of aryl hydrocarbon receptor as a mediator of osteogenic action. J Bone Miner Res 26(9):2096–2111
Bachagol D et al (2018) Stimulation of liver IGF-1 expression promotes peak bone mass achievement in growing rats: a study with pomegranate seed oil. J Nutr Biochem 52:18–26
Pahwa H, Khan MT, Sharan K (2020) Hyperglycemia impairs osteoblast cell migration and chemotaxis due to a decrease in mitochondrial biogenesis. Mol Cell Biochem 469:109–118
Gavva C et al (2020) Glycosaminoglycans from fresh water fish processing discard—isolation, structural characterization, and osteogenic activity. Int J Biol Macromol 145:558–567
Khan K et al (2012) [6]-Gingerol induces bone loss in ovary intact adult mice and augments osteoclast function via the transient receptor potential vanilloid 1 channel. Mol Nutr Food Res 56(12):1860–1873
Kureel J et al (2014) miR-542-3p suppresses osteoblast cell proliferation and differentiation, targets BMP-7 signaling and inhibits bone formation. Cell Death Dis 5:e1050
Kushwaha P et al (2016) MicroRNA 874-3p exerts skeletal anabolic effects epigenetically during weaning by suppressing Hdac1 expression. J Biol Chem 291(8):3959–3966
Hyde NK et al (2017) Maternal nutrition during pregnancy: intake of nutrients important for bone health. Matern Child Health J 21(4):845–851
Switkowski KM et al (2016) Maternal protein intake during pregnancy and linear growth in the offspring. Am J Clin Nutr 104(4):1128–1136
Walther T et al (2011) High-protein diet in lactation leads to a sudden infant death-like syndrome in mice. PLoS ONE 6(3):e17443
Dirckx N, Van Hul M, Maes C (2013) Osteoblast recruitment to sites of bone formation in skeletal development, homeostasis, and regeneration. Birth Defects Res C Embryo Today 99(3):170–191
Karner CM, Long F (2018) Glucose metabolism in bone. Bone 115:2–7
Maredsous C et al (2016) High-protein exposure during gestation or lactation or after weaning has a period-specific signature on rat pup weight, adiposity, food intake, and glucose homeostasis up to 6 weeks of age. J Nutr 146(1):21–29
Carlin G et al (2020) Perinatal exposure of rats to a maternal diet with varying protein quantity and quality affects the risk of overweight in female adult offspring. J Nutr Biochem 79:108333
Carlin G et al (2019) Maternal high-protein diet during pregnancy modifies rat offspring body weight and insulin signalling but not macronutrient preference in adulthood. Nutrients 11(1):96
Kenkre JS, Bassett J (2018) The bone remodelling cycle. Ann Clin Biochem 55(3):308–327
Tanaka S (2007) Signaling axis in osteoclast biology and therapeutic targeting in the RANKL/RANK/OPG system. Am J Nephrol 27(5):466–478
Jensen ED, Gopalakrishnan R, Westendorf JJ (2010) Regulation of gene expression in osteoblasts. BioFactors 36(1):25–32
Deodati A, Inzaghi E, Cianfarani S (2019) Epigenetics and in utero acquired predisposition to metabolic disease. Front Genet 10:1270
Gicquel C, El-Osta A, Le Bouc Y (2008) Epigenetic regulation and fetal programming. Best Pract Res Clin Endocrinol Metab 22(1):1–16
Dumortier O et al (2014) Maternal protein restriction leads to pancreatic failure in offspring: role of misexpressed microRNA-375. Diabetes 63(10):3416–3427
Alejandro EU et al (2014) Maternal diet-induced microRNAs and mTOR underlie beta cell dysfunction in offspring. J Clin Invest 124(10):4395–4410
Zhang J et al (2009) Maternal high fat diet during pregnancy and lactation alters hepatic expression of insulin like growth factor-2 and key microRNAs in the adult offspring. BMC Genomics 10:478
Wang RN et al (2014) Bone morphogenetic protein (BMP) signaling in development and human diseases. Genes Dis 1(1):87–105
Lee KS et al (2000) Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol 20(23):8783–8792
Karner CM, Lee SY, Long F (2017) Bmp induces osteoblast differentiation through both Smad4 and mTORC1 signaling. Mol Cell Biol 37(4):e00253-16
Nishimura R et al (1998) Smad5 and DPC4 are key molecules in mediating BMP-2-induced osteoblastic differentiation of the pluripotent mesenchymal precursor cell line C2C12. J Biol Chem 273(4):1872–1879
Yamamoto N et al (1997) Smad1 and smad5 act downstream of intracellular signalings of BMP-2 that inhibits myogenic differentiation and induces osteoblast differentiation in C2C12 myoblasts. Biochem Biophys Res Commun 238(2):574–580
Acknowledgements
We thank Dr. Naibedya Chattopadhyay (CSIR-Central Drug Research Institute, Lucknow, India) for the µ-CT facility and Genotypic Technology Private Ltd. (Bangalore, India) for the small RNA sequencing and analysis.
Funding
This study was supported by Department of Biotechnology, Government of India. Funding from the Science and Engineering Research Board (SERB), Government of India (KS), and research fellowship grants from the Department of Science and Technology (GE) are also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest in regard to this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ellur, G., Sukhdeo, S.V., Khan, M.T. et al. Maternal high protein-diet programs impairment of offspring’s bone mass through miR-24-1-5p mediated targeting of SMAD5 in osteoblasts. Cell. Mol. Life Sci. 78, 1729–1744 (2021). https://doi.org/10.1007/s00018-020-03608-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-020-03608-6