Abstract
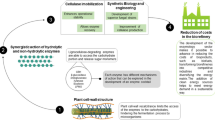
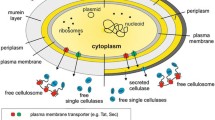
Dissociative enzymes such as cellulases are greatly desired for a variety of applications in the food, fuel, and fiber industries. Cellulases and other cell wall–degrading enzymes are currently being engineered with improved traits for application in the breakdown of lignocellulosic biomass. Biochemical assays using these “designer” enzymes have traditionally been carried out using synthetic substrates such as crystalline bacterial microcellulose (BMCC). However, the use of synthetic substrates may not reflect the actual action of these cellulases on real plant biomass. We examined the potential of suspension cell walls from several plant species as possible alternatives for synthetic cellulose substrates. Suspension cells grow synchronously; hence, their cell walls are more uniform than those derived from mature plants. This work will help to establish a new assay system that is more genuine than using synthetic substrates. In addition to this, we have demonstrated that it is feasible to produce cellulases inexpensively and at high concentrations and activities in plants using a recombinant plant virus expression system. Our long-term goals are to use this system to develop tailored cocktails of cellulases that have been engineered to function optimally for specific tasks (i.e., the conversion of biomass into biofuel or the enhancement of nutrients available in livestock feed). The broad impact would be to provide a facile and economic system for generating industrial enzymes that offer green solutions to valorize biomass in industrialized communities and specifically in developing countries.





Similar content being viewed by others
References
Bhat, M. (2000). Cellulases and related enzymes in biotechnology. Biotechnology Advances, 18(5), 355–383.
Park, S. H., Ong, R. G., & Sticklen, M. (2016 Jun). Strategies for the production of cell wall-deconstructing enzymes in lignocellulosic biomass and their utilization for biofuel production. Plant Biotechnology Journal, 14(6), 1329–1344.
Ruth, M.; Mai, T.; Newes, E.; Aden, A.; Warner, E.; Uriarte, C; Inman, D.; Simpkins, T.; Argo, A. (0DUFK 2013). Projected biomass utilization for fuels and power in a mature market. Transportation Energy Futures Series. Prepared for the U.S. Department of Energy by National Renewable Energy Laboratory, Golden, CO. DOE/GO-102013-3707. 153 pp.
Johnson, E. (2016). Integrated enzyme production lowers the cost of cellulosic ethanol. Biofuels Bioproducts and Biorefining, 10(2), 164–174.
Wilson, D. B. (2004). Studies of Thermobifida fusca plant cell wall degrading enzymes. Chemical Record, 4(2), 72–82.
Sticklen, M. B. (2008). Plant genetic engineering for biofuel production: Towards affordable cellulosic ethanol. Nature Reviews. Genetics, 9(6), 433–443.
Kostylev, M., & Wilson, D. (2012). Synergistic interactions in cellulose hydrolysis. Biofuels, 3(1), 61–70.
Jalak, J., Kurašin, M., Teugjas, H., & Väljamäe, P. (2012). Endo-exo synergism in cellulose hydrolysis revisited. The Journal of Biological Chemistry, 287(34), 28802–28815.
Santos, R. B., Abranches, R., Fischer, R., Sack, M., & Holland, T. (2016). Putting the spotlight back on plant suspension cultures. Frontiers in Plant Science, 7, 297. Published online 2016 Mar 11. https://doi.org/10.3389/fpls.2016.00297.
Twyman, R. M., Stoger, E., Schillberg, S., Christou, P., & Fischer, R. (2003). Molecular farming in plants: Host ¨ systems and expression technology. Trends in Biotechnology, 1, 570–578.
Wong-Arce, A., González-Ortega, O., & Rosales-Mendoza, S. (2017). Plant-made vaccines in the fight against cancer. Trends in Biotechnology, 35(3), 241–256.
Holtz, B. R., Berquist, B. R., Bennett, L. D., Kommineni, V. J. M., Munigunti, R. K., White, E. L., Wilkerson, D. C., Wong, K. Y. I., Ly, L. H., & Marcel, S. (2015). Commercial-scale biotherapeutics manufacturing facility for plant-made pharmaceuticals. Plant Biotechnology Journal, 13(8), 1180–1190.
Hefferon, K. (2017). Plant virus expression vectors: A powerhouse for global health. Biomedicines, 5(3), 44. Published online 2017 Jul 30. https://doi.org/10.3390/biomedicines5030044.
Tschofen, M., Knopp, D., Hood, E., & Stoger, E. (2016). Plant molecular farming: Much more than medicines annual rev. Analytical Chemistry, 9, 271–294.
Wu, J., Wang, M., Zhang, H., & Liu, R. (2015). Extreme thermophilic enzyme CelB-m efficiently degrades the cellulose in transgenic Arabidopsis thaliana. Applied Biochemistry and Biotechnology, 177(2), 362–372.
Tomassetti, S., Pontiggia, D., Verrascina, I., Reca, I. B., Francocci, F., Salvi, G., Cervone, F., & Ferrari, S. (2015). Controlled expression of pectic enzymes in Arabidopsis thaliana enhances biomass conversion without adverse effects on growth. Phytochemistry, 112, 221–230.
Ransom, C., Balan, V., Biswas, G., Dale, B., Crockett, E., & Sticklen, M. (2007). Heterologous Acidothermus cellulolyticus 1,4-beta-endoglucanase E1 produced within the corn biomass converts corn stover into glucose. Applied Biochemistry and Biotechnology, 137–140(1–12), 207–219.
Min, D., Li, Q., Jameel, H., Chiang, V., & Chang, H. M. (2012). The cellulase-mediated saccharification on wood derived from transgenic low-lignin lines of black cottonwood (Populus trichocarpa). Applied Biochemistry and Biotechnology, 168(4), 947–955.
Jung, S.-K., Parisutham, V., Jeong, S. H., & Lee, S. K. (2012). Heterologous expression of plant cell wall degrading enzymes for effective production of cellulosic biofuels. Journal of Biomedicine & Biotechnology, 405842.
Jiang, X. R., Zhou, X. Y., Jiang, W. Y., Gao, X. R., & Li, W. L. (2011). Expressions of thermostable bacterial cellulases in tobacco plant. Biotechnology Letters, 33(9), 1797–1803.
Klinger, J., Fischer, R., & Commandeur, U. (2015). Comparison of Thermobifida fusca cellulases expressed in Escherichia coli and indicates advantages of the plant system for the expression of bacterial cellulases. Frontiers in Plant Science, 6, 1047.
Hood EE. Plant-based biofuels F1000Research 2016, 5(F1000 Faculty Rev):185.
Kostylev, M., Wilson, D. A distinct model of synergism between a processive endocellulase (TfCel9A) and an exocellulase (TfCel48A) from Thermobifida fusca. January 2014 Volume 80 Number 1 Applied and Environmental Microbiology p. 339–344.
Kruer-Zerhusen N, Cantero-Tubilla B, Wilson D. Characterization of cellulose crystallinity after enzymatic treatment using Fourier transform infrared spectroscopy (FTIR). Cellulose. 2017.
King, B. C., Donnelly, M. K., Bergstrom, G. C., Walker, L. P., & Gibson, D. M. (2009). An optimized microplate assay system for quantitative evaluation of plant cell wall-degrading enzyme activity of fungal culture extracts. Biotechnology and Bioengineering, 102(4), 1033–1044. https://doi.org/10.1002/bit.22151.
Cruys-Bagger, N., Elmerdahl, J., Praestgaard, E., Tatsumi, H., Spodsberg, N., Borch, K., & Westh, P. (2012). Pre-steady-state kinetics for hydrolysis of insoluble cellulose by cellobiohydrolase Cel7A. The Journal of Biological Chemistry, 287(22), 18451–18458. https://doi.org/10.1074/jbc.M111.334946 Epub 2012 Apr 9.
Hefferon, K. L., & Dugdale, B. G. (2003). Independent expression of Rep and RepA and their roles in regulating bean yellow dwarf virus replication. Journal of General Virology, 84(12), 3465–3472.
Hefferon, K. L., & Fan, Y. (2004). Expression of a vaccine protein in a plant cell line using a geminivirus-based replicon system. Vaccine., 23(3), 404–410.
Schmidt, J. A., McGrath, J. M., Hanson, M. R., Long, S. P., & Ahner, B. A. (2019). Field-grown tobacco plants maintain robust growth while accumulating large quantities of a bacterial cellulase in chloroplasts. Nature Plants, 5(7), 715–721. https://doi.org/10.1038/s41477-019-0467-z Epub 2019 Jul 8.
Nakahira, Y., Ishikawa, K., Tanaka, K., Tozawa, Y., & Shiina, T. (2013). Overproduction of hyperthermostable β-1,4-endoglucanase from the archaeon Pyrococcus horikoshii by tobacco chloroplast engineering. Bioscience, Biotechnology, and Biochemistry, 77(10), 2140–2143.
Longoni, P., Leelavathi, S., Doria, E., Reddy, V. S., & Cella, R. (2015). Production by tobacco transplastomic plants of recombinant fungal and bacterial cell-wall degrading enzymes to be used for cellulosic biomass saccharification. BioMed Research International, 289759.
Giritch, A., Klimyuk, V., & Gleba, Y. (2017). 125 years of virology and ascent of biotechnologies based on viral expression. Tsitologiia i Genetika, 51(2), 19–39.
Song, E. G., & Ryu, K. H. (2017). A pepper mottle virus-based vector enables systemic expression of endoglucanase D in non-transgenic plants. Archives of Virology, 162(12), 3717–3726.
Hood E, Requesens D. Commercial plant-produced recombinant cellulases for biomass conversion. Biotechnology in Agriculture and Forestry book series (AGRICULTURE, volume 68) 2014. , pp. 231–46.
Zhang, S., Barr, B. K., & Wilson, D. B. (2000). Effects of noncatalytic residue mutations on substrate specificity and ligand binding of Thermobifida fusca endocellulase cel6A. European Journal of Biochemistry, 267(1), 244–252.
Li, Q., Song, J., Peng, S., Wang, J. P., & Qu, G. Z. (2014). Plant biotechnology for lignocellulosic biofuel production. Plant Biotechnology Journal, 12(9), 1174–1192.
Kumari, U., Singh, R., Ray, T., Rana, S., Saha, P., Malhotra, K., & Daniell, H. (2019). Validation of leaf enzymes in the detergent and textile industries: Launching of a new platform technology. Plant Biotechnology Journal, 17(6), 1167–1182. https://doi.org/10.1111/pbi.13122 Epub 2019 Apr 23.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hefferon, K., Cantero-Tubilla, B., Badar , U. et al. Plant-Based Cellulase Assay Systems as Alternatives for Synthetic Substrates. Appl Biochem Biotechnol 192, 1318–1330 (2020). https://doi.org/10.1007/s12010-020-03395-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03395-7