Abstract
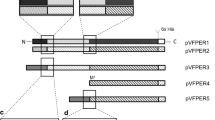
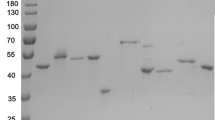
Pernisine is a subtilisin-like serine proteinase secreted by the hyperthermophilic archaeon Aeropyrum pernix. The significant properties of this proteinase are remarkable stability and ability to degrade the infectious prion proteins. Here we show the production of pernisine in the periplasm of Escherichia coli. This strategy prevented the aggregation of pernisine in the cytoplasm and increased the purity of the isolated pernisine. The thermostability of this recombinant pernisine was significantly increased compared with previous studies. In addition, several truncated pernisine variants were constructed and expressed in E. coli to identify the minimally active domain. The catalytic domain of pernisine consists of the αẞα structurally similar core flanked by the N-terminal and C-terminal outer regions. The deletion of the C-terminal α helix did not affect the pernisine activity at 90 °C. However, the complete deletion of the C-terminal outer region resulted in loss of proteolytic activity. The pernisine variant, in which the N-terminal outer region was deleted, had a reduced activity at 90 °C. These results underline the importance of the Ca2+ binding sites predicted in these outer regions for stability and activity of pernisine.
Key points
• Aggregation of produced pernisine was prevented by translocation into periplasm.
• Thermostability of mature pernisine was increased.
• The outer regions of the catalytic core are required for pernisine thermostability.







Similar content being viewed by others
Data availability
Authors can confirm that all relevant data are included in the article and its supplementary information file.
References
Bagos PG, Nikolaou EP, Liakopoulos TD, Tsirigos KD (2010) Combined prediction of Tat and Sec signal peptides with hidden Markov models. Bioinformatics 26(22):2811–2817
Bardy SL, Eichler J, Jarrell KF (2003) Archaeal signal peptides — a comparative survey at the genome level. Protein Sci 12(9):1833–1843. https://doi.org/10.1110/ps.03148703
Bentley WE, Mirjalili N, Andersen DC, Davis RH, Kompala DS (1990) Plasmid-encoded protein: the principal factor in the “metabolic burden” associated with recombinant bacteria. Biotechnol Bioeng 35:668–681. https://doi.org/10.1002/bit.260350704
Carneiro S, Ferreira EC, Rocha I (2013) Metabolic responses to recombinant bioprocesses in Escherichia coli. J Biotechnol 164(3):396–408. https://doi.org/10.1016/j.jbiotec.2012.08.026
Catara G, Ruggiero G, La Cara F, Digilio FA, Capasso A, Rossi M (2003) A novel extracellular subtilisin-like protease from the hyperthermophile Aeropyrum pernix K1: biochemical properties, cloning, and expression. Extremophiles 7(5):391–399. https://doi.org/10.1007/s00792-003-0337-4
Choi JH, Lee SY (2004) Secretory and extracellular production of recombinant proteins using Escherichia coli. Appl Microbiol Biotechnol 64(5):625–635. https://doi.org/10.1007/s00253-004-1559-9
Crane JM, Randall LL (2017) The Sec system: protein export in Escherichia coli. EcoSal Plus 7(2):1–73. https://doi.org/10.1128/ecosalplus.ESP-0002-2017
Croocker PC, Yoshihiko C, Uchida A (1999) Purification and characterization of an intracellular heat-stable proteinase (pernilase) from the marine hyperthermophilic archaeon Aeropyrum pernix K1. Extremophiles 3:3–9. https://doi.org/10.1007/s007920050093
Eggen R, Geerling A, Watts J, de Vos WM (1990) Characterization of pyrolysin, a hyperthermoactive serine protease from the archaebacterium Pyrococcus furiosus. FEMS Microbiol Lett 71(1–2):17–20. https://doi.org/10.1111/j.1574-6968.1990.tb03791.x
Fang N, Zhong C, Liang X, Tang X, Tang B (2010) Improvement of extracellular production of a thermophilic subtilase expressed in Escherichia coli by random mutagenesis of its N-terminal propeptide. Appl Microbiol Biotechnol 85:1473–1481. https://doi.org/10.1007/s00253-009-2183-5
Glick BR (1995) Metabolic load and heterologous gene expression. Biotechnol Adv 13(2):247–261. https://doi.org/10.1016/0734-9750(95)00004-A
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14(1):33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Ikemura H, Takagi H, Inouye M (1987) Requirement of pro-sequence for the production of active subtilisin E. J Biol Chem 262(16):7859–7864
Jain SC, Shinde U, Li Y, Inouye M, Berman HM (1998) The crystal structure of an autoprocessed Ser221Cys-subtilisin E-propeptide complex at 2.0 Å resolution. J Mol Biol 284(1):137–144. https://doi.org/10.1006/jmbi.1998.2161
Li Y, Inouye M (1994) Autoprocessing of prothiolsubtilisin E in which active-site serine 221 is altered to cysteine. J Biol Chem 269(6):4169–4174
Li Y, Hu Z, Jordan F, Inouye M (1995) Functional analysis of the propeptide of subtilisin e as an intramolecular chaperone for protein folding. J Biol Chem 270(42):25127–25132. https://doi.org/10.1074/jbc.270.42.25127
Ohta Y, Hojo H, Aimoto S, Kobayashi T, Zhu X, Jordan F, Inouye M (1991) Pro-peptide as an intermolecular chaperone: renaturation of denatured subtilisin E with a synthetic pro-peptide. Mol Microbiol 5:1507–1510. https://doi.org/10.1111/j.1365-2958.1991.tb00797.x
Pulido M, Saito K, Tanaka S, Koga Y, Morikawa M, Takano K, Kanaya S (2006) Ca2+-dependent maturation of subtilisin from a hyperthermophilic archaeon, Thermococcus kodakaraensis: the propeptide is a potent inhibitor of the mature domain but is not required for its folding. Appl Environ Microbiol 72(6):4154–4162. https://doi.org/10.1128/AEM.02696-05
Sako Y, Nomura N, Uchida A, Ishida Y, Morii H, Koga Y, Hoaki T, Maruyama T (1996) Aeropyrum pemix gen. nov., sp. nov., a novel aerobic hyperthermophilic archaeon growing at temperatures up to 100°C. Int J Syst Bacteriol 46(4):1070–1077. https://doi.org/10.1099/00207713-46-4-1070
Sako Y, Croocker PC, Ishida Y (1997) An extremely heat-stable extracellular proteinase (aeropyrolysin) from the hyperthermophilic archaeon Aeropyrum pernix Kl. FEBS Lett 415(3):329–334. https://doi.org/10.1016/S0014-5793(97)01153-8
Shinde U, Inouye M (1995) Folding pathway mediated by an intramolecular chaperone: characterization of the structural changes in pro-subtilisin E coincident with autoprocessing. J Mol Biol 252(1):25–30. https://doi.org/10.1006/jmbi.1995.0472
Shinde U, Inouye M (2000) Intramolecular chaperones: polypeptide extensions that modulate protein folding. Semin Cell Dev Biol 11(1):35–44. https://doi.org/10.1006/scdb.1999.0349
Škrlj N, Erčulj N, Dolinar M (2009) A versatile bacterial expression vector based on the synthetic biology plasmid pSB1. Protein Expr Purif 64(2):198–204. https://doi.org/10.1016/j.pep.2008.10.019
Šnajder M, Vilfan T, Maja Č, Rupreht R, Popović M, Juntes P, Šerbec VČ, Ulrih NP (2012) Enzymatic degradation of PrPSc by a protease secreted from Aeropyrum pernix K1. PLoS One 7(6):e39548. https://doi.org/10.1371/journal.pone.0039548
Šnajder M, Mihelič M, Turk D, Ulrih NP (2015) Codon optimisation is key for pernisine expression in Escherichia coli. PLoS One 10:e0123288. https://doi.org/10.1371/journal.pone.0123288
Šnajder M, Rincón AFC, Magdevska V, Bahun M, Kranjc L, Paš M, Juntes P, Petković H, Ulrih NP (2019) Extracellular production of the engineered thermostable protease pernisine from Aeropyrum pernix K1 in Streptomyces rimosus. Microb Cell Factories 18:196. https://doi.org/10.1186/s12934-019-1245-3
Strausberg S, Alexander P, Wang L, Schwarz F, Bryan P (1993) Catalysis of protein folding reaction: thermodynamic and kinetic analysis of subtilisin BPN’ interactions with its propeptide fragment. Biochemistry 32:8112–8119. https://doi.org/10.1021/bi00083a009
Subbian E, Yabuta Y, Shinde UP (2005) Folding pathway mediated by an intramolecular chaperone: intrinsically unstructured propeptide modulates stochastic activation of subtilisin. J Mol Biol 347(2):367–383. https://doi.org/10.1016/j.jmb.2005.01.028
Takeuchi Y, Tanaka S, Matsumura H, Koga Y, Takano K, Kanaya S (2009) Requirement of a unique Ca2+-binding loop for folding of Tk-subtilisin from a hyperthermophilic archaeon. Biochemistry 48(44):10637–10643. https://doi.org/10.1021/bi901334b
Tanaka S, Matsumura H, Koga Y, Takano K, Kanaya S (2007a) Four new crystal structures of Tk-subtilisin in unautoprocessed, autoprocessed and mature forms: insight into structural changes during maturation. J Mol Biol 372(4):1055–1069. https://doi.org/10.1016/j.jmb.2007.07.027
Tanaka S, Saito K, Chon H, Matsumura H, Koga Y, Takano K, Kanaya S (2007b) Crystal structure of unautoprocessed precursor of subtilisin from a hyperthermophilic archaeon: evidence for Ca2+-induced folding. J Biol Chem 282(11):8246–8255. https://doi.org/10.1074/jbc.M610137200
Tanaka S, Takeuchi Y, Matsumura H, Koga Y, Takano K, Kanaya S (2008) Crystal structure of Tk-subtilisin folded without propeptide: requirement of propeptide for acceleration of folding. FEBS Lett 582(28):3875–3878. https://doi.org/10.1016/j.febslet.2008.10.025
Uehara R, Takeuchi Y, Tanaka S, Takano K, Koga Y, Kanaya S (2012) Requirement of Ca2+ ions for the hyperthermostability of Tk-subtilisin from Thermococcus kodakarensis. Biochemistry 51(26):5369–5378. https://doi.org/10.1021/bi300427u
Vieille C, Zeikus GJ (2001) Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol Mol Biol Rev 65(1):1–43. https://doi.org/10.1128/MMBR.65.1.1-43.2001
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46(W1):W296–W303. https://doi.org/10.1093/nar/gky427
Yabuta Y, Takagi H, Inouye M, Shinde U (2001) Folding pathway mediated by an intramolecular chaperone: propeptide release modulates activation precision of pro-subtilisin*. J Biol Chem 276(48):44427–44434. https://doi.org/10.1074/jbc.M107573200
Yabuta Y, Subbian E, Takagi H, Shinde U, Inouye M (2002) Folding pathway mediated by and intramolecular chaperone: dissecting conformational changes coincident with autoprocessing and the role of Ca2+ in subtilisin maturation. J Biochem 131:31–37. https://doi.org/10.1093/oxfordjournals.jbchem.a003074
Acknowledgments
We are thankful to Marko Dolinar for providing the pMD204 plasmid.
Funding
This work was supported by the Slovenian Research Agency through grant L7-8277 (to NPU) and postgraduate research funding (to MB). We are also thankful to Borer Chemie AG for financial support through the L7-8722 grant.
Author information
Authors and Affiliations
Contributions
MB and NPU designed research. MB and KH conducted experiments. MB analyzed data and wrote the manuscript. NPU contributed materials/ analytical tools and coordinated the project. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethic approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 499 kb)
Rights and permissions
About this article
Cite this article
Bahun, M., Hartman, K. & Poklar Ulrih, N. Periplasmic production of pernisine in Escherichia coli and determinants for its high thermostability. Appl Microbiol Biotechnol 104, 7867–7878 (2020). https://doi.org/10.1007/s00253-020-10791-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10791-w