Abstract
Purpose
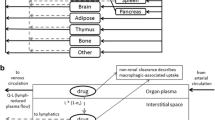
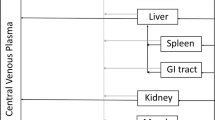
The purpose of this work was to investigate the role of the lymphatic system in the pharmacokinetics of etanercept, a fusion protein.
Methods
Etanercept 1 mg/kg was administered intravenously (IV) and subcutaneously (SC) to thoracic lymph duct-cannulated and sham-operated control rats. Blood and lymph samples were obtained for up to 6 days.
Results
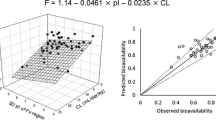
Model-based SC bioavailability of etanercept was 65.2% in the control group. In lymph-cannulated rats, etanercept concentration in the lymph was consistently lower than in serum following IV dosing; and the concentration in the lymph was significantly higher than in serum after SC injection. The absorption occurred predominantly through the lymphatic pathway (82.7%), and only 17.3% by direct uptake into the central compartment (blood pathway). Lymphatic cannulation reduced the area under the serum concentration-time curve by 28% in IV group and by 91% in SC group. A mechanistic pharmacokinetic model that combined dual absorption pathways with redistribution of the systemically available protein drug into lymph was developed. The model successfully captured serum and lymph data in all groups simultaneously, and all parameters were estimated with sufficient precision.
Conclusions
Lymphatic system was shown to play an essential role in systemic disposition and SC absorption of etanercept.



Similar content being viewed by others
References
de la Torre BG, Albericio F. The Pharmaceutical Industry in 2018. An analysis of FDA drug approvals from the perspective of molecules. Molecules. 2019;24(4).
Onkar Sumant SS. protein therapeutics market overview. Allied market research. 2017.
Zou Y, Bateman TJ, Adreani C, Shen X, Cunningham PK, Wang B, et al. Lymphatic absorption, metabolism, and excretion of a therapeutic peptide in dogs and rats. Drug Metab Dispos. 2013;41(12):2206–14.
Kagan L. Pharmacokinetic modeling of the subcutaneous absorption of therapeutic proteins. Drug Metab Dispos. 2014;42(11):1890–905.
Dahlberg AM, Kaminskas LM, Smith A, Nicolazzo JA, Porter CJ, Bulitta JB, et al. The lymphatic system plays a major role in the intravenous and subcutaneous pharmacokinetics of trastuzumab in rats. Mol Pharm. 2014;11(2):496–504.
Glassman PM, Balthasar JP. Physiologically-based modeling of monoclonal antibody pharmacokinetics in drug discovery and development. Drug Metab Pharmacokinet. 2019;34(1):3–13.
Varkhede N, Forrest ML. Understanding the Monoclonal Antibody Disposition after Subcutaneous Administration using a Minimal Physiologically based Pharmacokinetic Model. J Pharm Pharm Sci. 2018;21(1s):130s–48s.
Chan LJ, Bulitta JB, Ascher DB, Haynes JM, McLeod VM, Porter CJ, et al. PEGylation does not significantly change the initial intravenous or subcutaneous pharmacokinetics or lymphatic exposure of trastuzumab in rats but increases plasma clearance after subcutaneous administration. Mol Pharm. 2015;12(3):794–809.
Styles IK, Feeney OM, Nguyen TH, Brundel DHS, Kang DW, Clift R, et al. Removal of interstitial hyaluronan with recombinant human hyaluronidase improves the systemic and lymphatic uptake of cetuximab in rats. J Control Release. 2019;315:85–96.
Richter W, Gruyer M, Birnböck H. Marked contribution of lymphatic route in the SC absorption of an IgG fusion protein in the rabbit. In.AAPS National Biotech Conference 2014; 2014. p. 19–21.
Peppel K, Crawford D, Beutler B. A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. J Exp Med. 1991;174(6):1483–9.
Feldmann M, Maini RN. Lasker clinical medical research award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med. 2003;9(10):1245–50.
Lon HK, Liu D, Zhang Q, DuBois DC, Almon RR, Jusko WJ. Pharmacokinetic-pharmacodynamic disease progression model for effect of etanercept in Lewis rats with collagen-induced arthritis. Pharm Res. 2011;28(7):1622–30.
Chen X, DuBois DC, Almon RR, Jusko WJ. Biodistribution of etanercept to tissues and sites of inflammation in arthritic rats. Drug Metab Dispos. 2015;43(6):898–907.
Kagan L, Gershkovich P, Mendelman A, Amsili S, Ezov N, Hoffman A. The role of the lymphatic system in subcutaneous absorption of macromolecules in the rat model. Eur J Pharm Biopharm. 2007;67(3):759–65.
Ionac M. One technique, two approaches, and results: thoracic duct cannulation in small laboratory animals. Microsurgery. 2003;23(3):239–45.
Milling SW, Jenkins C, MacPherson G. Collection of lymph-borne dendritic cells in the rat. Nat Protoc. 2006;1(5):2263–70.
Kagan L, Turner MR, Balu-Iyer SV, Mager DE. Subcutaneous absorption of monoclonal antibodies: role of dose, site of injection, and injection volume on rituximab pharmacokinetics in rats. Pharm Res. 2012;29(2):490–9.
Radwanski E, Chakraborty A, Van Wart S, Huhn RD, Cutler DL, Affrime MB, et al. Pharmacokinetics and leukocyte responses of recombinant human interleukin-10. Pharm Res. 1998;15(12):1895–901.
Kota J, Machavaram KK, McLennan DN, Edwards GA, Porter CJ, Charman SA. Lymphatic absorption of subcutaneously administered proteins: influence of different injection sites on the absorption of darbepoetin alfa using a sheep model. Drug Metab Dispos. 2007;35(12):2211–7.
McLennan DN, Porter CJ, Edwards GA, Martin SW, Heatherington AC, Charman SA. Lymphatic absorption is the primary contributor to the systemic availability of epoetin Alfa following subcutaneous administration to sheep. J Pharmacol Exp Ther. 2005;313(1):345–51.
Kagan L, Mager DE. Mechanisms of subcutaneous absorption of rituximab in rats. Drug Metab Dispos. 2013;41(1):248–55.
Acknowledgments
The authors would like to thank Dr. John Harrold for development and continuous support of the Ubiquity modeling framework. The study was supported in part by a grant from Bristol Myers Squibb to Leonid Kagan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gao, X., Voronin, G., Generaux, C. et al. Lymphatic Distribution of Etanercept Following Intravenous and Subcutaneous Delivery to Rats. Pharm Res 37, 155 (2020). https://doi.org/10.1007/s11095-020-02860-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02860-6