Abstract
Purpose
To efficiently develop a tablet formulation of carbamazepine using a soluble cocrystal with excess coformer to maintain phase stability during dissolution.
Methods
The carbamazepine – glutaric acid cocrystal (CBZ-GLA, 1:1) and excess glutaric acid (GLA) were mixed with suitable tablet excipients, which were selected to address powder flowability and tabletability deficiencies specifically. Tablet friability and dissolution profiles were evaluated to guide formulation optimization. Dry granules were prepared by milling simulated ribbons.
Results
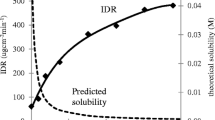
A binary blend of CBZ-GLA and GLA had poor flowability and marginal tabletability. Therefore, silica coated Avicel PH-102 (sMCC) was applied as a binder to improve the flow property and tabletability. A formulation consisting of sMCC, CBZ-GLA, and GLA exhibited good manufacturability but did not show improved dissolution because of rapid precipitation of CBZ dihydrate when CBZ-GLA came in contact with water. Dry granulation of CBZ-GLA and GLA dramatically improved dissolution profile due to the intimate contact between CBZ-GLA and GLA. Such cocrystal - coformer granules also led to much improved tablet manufacturability and dissolution.
Conclusion
The successful tablet development of CBZ-GLA, using < 3 g of the cocrystal in <3 weeks, demonstrates an efficient workflow for tablet formulation development based on material-sparing and predictive powder characterization techniques. This workflow is useful for early tablet development using enabling solid form, such as cocrystal, when only a small amount of material is available.







Similar content being viewed by others
Abbreviations
- CBZ-GLA :
-
Carbamazepine glutaric acid cocrystal
- CBZ :
-
Carbamazepine
- GLA :
-
Glutaric acid
- PXRD :
-
Powder X-ray diffraction
References
Leuenberger H, Lanz M. Pharmaceutical powder technology - from art to science: the challenge of the FDA’s process analytical technology initiative. Adv Powder Technol. 2005;16(1):3–25.
Sun CC. Materials science tetrahedron-a useful tool for pharmaceutical research and development. J Pharm Sci. 2009;98(5):1671–87.
FDA. Final Report on Pharmaceutical cGMPs for the 21st Century—A Risk-Based Approach. Available from: http://www.fda.gov/cder/gmp/gmp2004/GMP_finalreport2004.htm.
FDA. Critical path opportunities for generic drugs. Available from: http://www.fda.gov/oc/initiatives/criticalpath/reports/generic.html.
Craig DQM. Pharmaceutical materials science - resuscitation or reincarnation? J Pharm Pharmacol. 1997;49(2):119–26.
Cui Y. A material science perspective of pharmaceutical solids. Int J Pharm. 2007;339(1–2):3–18.
Wassgren C, Curtis JS. The application of computational modeling to pharmaceutical materials science. MRS Bull. 2006;31(11):900–4.
Shah P. Use of nanotechnologies for drug delivery. MRS Bull. 2006;31(11):894–9.
Sun CC, Hou H, Gao P, Ma C, Medina C, Alvarez FJ. Development of a high drug load tablet formulation based on assessment of powder manufacturability: moving towards quality by design. J Pharm Sci. 2009;98(1):239–47.
Osei-Yeboah F, Sun CC. Validation and applications of an expedited tablet friability method. Int J Pharm. 2015;484(1–2):146–55.
Sun CC. Setting the bar for powder flow properties in successful high speed tableting. Powder Technol. 2010;201(1):106–8.
Serajuddin ATM. Salt formation to improve drug solubility. Adv Drug Del Rev. 2007;59(7):603–16.
Konno H, Handa T, Alonzo DE, Taylor LS. Effect of polymer type on the dissolution profile of amorphous solid dispersions containing felodipine. Eur J Pharm Biopharm. 2008;70(2):493–9.
Demuth B, Nagy ZK, Balogh A, Vigh T, Marosi G, Verreck G, et al. Downstream processing of polymer-based amorphous solid dispersions to generate tablet formulations. Int J Pharm. 2015;486(1–2):268–86.
Duggirala NK, Perry ML, Almarsson O, Zaworotko MJ. Pharmaceutical cocrystals: along the path to improved medicines. Chem Commun. 2016;52(4):640–55.
Tajarobi F, Larsson A, Matic H, Abrahmsen-Alami S. The influence of crystallization inhibition of HPMC and HPMCAS on model substance dissolution and release in swellable matrix tablets. Eur J Pharm Biopharm. 2011;78(1):125–33.
Yamashita H, Sun CQC. Self-templating accelerates precipitation of carbamazepine dihydrate during the dissolution of a soluble carbamazepine cocrystal. Cryst Eng Comm. 2017;19(8):1156–9.
Yamashita H, Sun CC. Harvesting potential dissolution advantages of soluble cocrystals by depressing precipitation using the common coformer effect. Cryst Growth Des. 2016;16(12):6719–21.
Yamashita H, Sun CC. Improving dissolution rate of carbamazepine-glutaric acid cocrystal through solubilization by excess coformer. Pharm Res. 2018;35(1):4.
Yamashita H, Sun CC. Expedited tablet formulation development of a highly soluble carbamazepine cocrystal enabled by precipitation inhibition in diffusion layer. Pharm Res. 2019;36(6):90.
Childs SL, Rodriguez-Hornedo N, Reddy LS, Jayasankar A, Maheshwari C, McCausland L, et al. Screening strategies based on solubility and solution composition generate pharmaceutically acceptable cocrystals of carbamazepine. Cryst Eng Comm. 2008;10(7):856–64.
Hou H, Sun CC. Quantifying effects of particulate properties on powder flow properties using a ring shear tester. J Pharm Sci. 2008;97(9):4030–9.
Sun CC. Quantifying effects of moisture content on flow properties of microcrystalline cellulose using a ring shear tester. Powder Technol. 2016;289:104–8.
Zhou Q, Shi LM, Chattoraj S, Sun CC. Preparation and characterization of surface-engineered coarse microcrystalline cellulose through dry coating with silica nanoparticles. J Pharm Sci. 2012;101(11):4258–66.
USP. General Chapters In.Tablet friability. Rockville, MD United States Pharmacopoeial Convention; 2014.
JP. G6: Tablet friability test. In. Japan: Minister of Health, Labour and Welfare; 2011.
Ph. Eur. 2.9.7: Friability of uncoated tablets. In.: European Directorate for the Quality of Medicine and Health (EDQM); 2013.
Banga S, Chawla G, Varandani D, Mehta BR, Bansal AK. Modification of the crystal habit of celecoxib for improved processability. J Pharm Pharmacol. 2007;59(1):29–39.
Thakur A, Thipparaboina R, Kumar D, Gouthami KS, Shastri NR. Crystal engineered albendazole with improved dissolution and material attributes. Cryst Eng Comm. 2016;18(9):1489–94.
Garekani HA, Sadeghi F, Badiee A, Mostafa SA, Rajabi-Siahboomi AR. Crystal habit modifications of ibuprofen and their physicomechanical characteristics. Drug Dev Ind Pharm. 2001;27(8):803–9.
Kawashima Y, Imai A, Takeuchi H, Yamamoto H, Kamiya K, Hino T. Improved flowability and compactibility of spherically agglomerated crystals of ascorbic acid for direct tableting designed by spherical crystallization process. Powder Technol. 2003;130(1–3):283–9.
Kawashima Y, Okumura M, Takenaka H. Spherical crystallization - direct spherical agglomeration of salicylic-acid crystals during crystallization. Science. 1982;216(4550):1127–8.
Kumar S, Chawla G, Bansal AK. Spherical crystallization of mebendazole to improve processability. Pharm Dev Technol. 2008;13(6):559–68.
Katta J, Rasmuson AC. Spherical crystallization of benzoic acid. Int J Pharm. 2008;348(1–2):61–9.
Chattoraj S, Shi LM, Sun CC. Profoundly improving flow properties of a cohesive cellulose powder by surface coating with nano-silica through comilling. J Pharm Sci. 2011;100(11):4943–52.
Huang ZH, Scicolone JV, Gurumuthy L, Dave RN. Flow and bulk density enhancements of pharmaceutical powders using a conical screen mill: a continuous dry coating device. Chem Eng Sci. 2015;125:209–24.
Huang ZG, Scicolone JV, Han X, Dave RN. Improved blend and tablet properties of fine pharmaceutical powders via dry particle coating. Int J Pharm. 2015;478(2):447–55.
Ramlakhan M, Wu CY, Watano S, Dave RN, Pfeffer R. Dry particle coating using magnetically assisted impaction coating: modification of surface properties and optimization of system and operating parameters. Powder Technol. 2000;112(1–2):137–48.
Zhou Q, Qu L, Gengenbach T, Denman JA, Larson I, Stewart PJ, et al. Investigation of the extent of surface coating via mechanofusion with varying additive levels and the influences on bulk powder flow properties. Int J Pharm. 2011;413(1–2):36–43.
Sun CC. A classification system for tableting behaviors of binary powder mixtures. Asian J Pharm Sci. 2016;11(4):486–91.
Sun CC, Himmelspach MW. Reduced tabletability of roller compacted granules as a result of granule size enlargement. J Pharm Sci. 2006;95(1):200–6.
Leung LY, Mao C, Pieters SR, Yang C-Y. A proposed complete methodology to predict gravity flow obstruction of pharmaceutical powders in drug product manufacturing. J Pharm Sci. 2019;108(1):464–75.
Dokoumetzidis A, Macheras P. A century of dissolution research: from Noyes and Whitney to the biopharmaceutics classification system. Int J Pharm. 2006;321(1–2):1–11.
Hawley M, Morozowich W. Modifying the diffusion layer of soluble salts of poorly soluble basic drugs to improve dissolution performance. Mol Pharm. 2010;7(5):1441–9.
Acknowledgements and Disclosures
Parts of this work were carried out in the Characterization Facility, University of Minnesota, a member of the NSF-funded Materials Research Facilities Network (http://www.mrfn.org) through the UMN MRSEC (DMR-1420013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yamashita, H., Sun, C.C. Material-Sparing and Expedited Development of a Tablet Formulation of Carbamazepine Glutaric Acid Cocrystal– a QbD Approach. Pharm Res 37, 153 (2020). https://doi.org/10.1007/s11095-020-02855-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02855-3