Abstract
Purpose
Lipid nanoparticles (SLN and NLC) were functionalized with the RVG29 peptide in order to target the brain and increase the neuronal uptake through the nicotinic acetylcholine receptors. These nanosystems were loaded with quercetin to take advantage of its neuroprotective properties mainly for Alzheimer’s disease.
Methods
The functionalization of nanoparticles with RVG29 peptide was confirmed by NMR and FTIR. Their morphology was assessed by transmission electron microscopy and nanoparticles size, polydispersity and zeta potential were determined by dynamic light scattering. The in vitro validation tests were conducted in hCMEC/D3 cells, a human blood-brain barrier model and thioflavin T binding assay was conducted to assess the process of amyloid-beta peptide fibrillation typical of Alzheimer’s disease.
Results
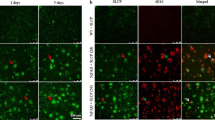
RVG29-nanoparticles displayed spherical morphology and size below 250 nm, which is compatible with brain applications. Zeta potential values were between −20 and −25 mV. Quercetin entrapment efficiency was generally higher than 80% and NLC nanoparticles were able to encapsulate up to 90%. The LDH assay showed that there is no cytotoxicity in hCMEC/D3 cell line and RVG29-nanoparticles clearly increased in 1.5-fold the permeability across the in vitro model of blood-brain barrier after 4 h of incubation compared with non-functionalized nanoparticles. Finally, this nanosystem was capable of inhibiting amyloid-beta aggregation in thioflavin T binding assay, suggesting its great potential for neuroprotection.
Conclusions
RVG29-nanoparticles that simultaneously target the blood-brain barrier and induce neurons protection against amyloid-beta fibrillation proved to be an efficient way of quercetin delivery and a promising strategy for future approaches in Alzheimer’s disease.

Graphical Abstract







Similar content being viewed by others
Data Availability
No raw/processed data is available.
References
Heoand HJ, Lee CY. Protective effects of quercetin and vitamin C against oxidative stress-induced neurodegeneration. J Agric Food Chem. 2004;52:7514–7.
Zhang ZJ, Cheang LC, Wang MW, Lee SM. Quercetin exerts a neuroprotective effect through inhibition of the iNOS/NO system and pro-inflammation gene expression in PC12 cells and in zebrafish. Int J Mol Med. 2011;27:195–203.
Dok-Go H, Lee KH, Kim HJ, Lee EH, Lee J, Song YS, et al. Neuroprotective effects of antioxidative flavonoids, quercetin, (+)-dihydroquercetin and quercetin 3-methyl ether, isolated from Opuntia ficus-indica var. saboten. Brain Res. 2003;965:130–6.
Sagara Y, Vanhnasy J, Maher P. Induction of PC12 cell differentiation by flavonoids is dependent upon extracellular signal-regulated kinase activation. J Neurochem. 2004;90:1144–55.
Min YD, Choi CH, Bark H, Son HY, Park HH, Lee S, et al. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-kappaB and p38 MAPK in HMC-1 human mast cell line. Inflamm Res: Official Journal of the European Histamine Research Society [et al]. 2007;56:210–5.
Kimata M, Shichijo M, Miura T, Serizawa I, Inagaki N, Nagai H. Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin Exp Allergy: J Br Soc Allergy Clin Immunol. 2000;30:501–8.
Afanas'ev IB, Dorozhko AI, Brodskii AV, Kostyuk VA, Potapovitch AI. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem Pharmacol. 1989;38:1763–9.
van Acker SA, van Balen GP, van den Berg DJ, Bast A, van der Vijgh WJ. Influence of iron chelation on the antioxidant activity of flavonoids. Biochem Pharmacol. 1998;56:935–43.
Ansari MA, Abdul HM, Joshi G, Opii WO, Butterfield DA. Protective effect of quercetin in primary neurons against Abeta(1-42): relevance to Alzheimer’s disease. J Nutr Biochem. 2009;20:269–75.
Kim H, Park BS, Lee KG, Choi CY, Jang SS, Kim YH, et al. Effects of naturally occurring compounds on fibril formation and oxidative stress of beta-amyloid. J Agric Food Chem. 2005;53:8537–41.
Maria S-GA, Ignacio M-MJ, Ramírez-Pineda Jose R, Marisol L-R, Edison O, Patricia C-GG. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology. 2015;93:134–45.
Guoand Y, Bruno RS. Endogenous and exogenous mediators of quercetin bioavailability. J Nutr Biochem. 2015;26:201–10.
Almeida AF, Borge GIA, Piskula M, Tudose A, Tudoreanu L, Valentova K, et al. Bioavailability of quercetin in humans with a focus on interindividual variation. Compr Rev Food Sci Food Saf. 2018;17:714–31.
Soni S, Ruhela RK, Medhi B. Nanomedicine in central nervous system (CNS) disorders: a present and future prospective. Adv Pharm Bull. 2016;6:319–35.
Chowdhury A, Kunjiappan S, Panneerselvam T, Somasundaram B, Bhattacharjee C. Nanotechnology and nanocarrier-based approaches on treatment of degenerative diseases. Int Nano Lett. 2017;7:91–122.
Le Novere N, Corringer PJ, Changeux JP. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol. 2002;53:447–56.
Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, et al. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78:703–11.
Hawkins BT, Egleton RD, Davis TP. Modulation of cerebral microvascular permeability by endothelial nicotinic acetylcholine receptors. Am J Physiol Heart Circ Physiol. 2005;289:H212–9.
Lafon M. Rabies virus receptors. J Neurovirol. 2005;11:82–7.
Kumar P, Wu H, McBride JL, Jung KE, Kim MH, Davidson BL, et al. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43.
Liu Y, Huang R, Han L, Ke W, Shao K, Ye L, et al. Brain-targeting gene delivery and cellular internalization mechanisms for modified rabies virus glycoprotein RVG29 nanoparticles. Biomaterials. 2009;30:4195–202.
Hua HC, Zhang XM, Mu HJ, Meng QQ, Jiang Y, Wang YY, et al. RVG29-modified docetaxel-loaded nanoparticles for brain-targeted glioma therapy. Int J Pharm. 2018;543:179–89.
You LH, Wang J, Liu TQ, Zhang YL, Han XX, Wang T, et al. Targeted brain delivery of rabies virus glycoprotein 29-modified deferoxamine-loaded nanoparticles reverses functional deficits in Parkinsonian mice. ACS Nano. 2018;12:4123–39.
Oswald M, Geissler S, Goepferich A. Targeting the central nervous system (CNS): a review of rabies virus-targeting strategies. Mol Pharm. 2017;14:2177–96.
Neves AR, Lucio M, Martins S, Lima JL, Reis S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int J Nanomedicine. 2013;8:177–87.
Neves AR, Queiroz JF, Reis S. Brain-targeted delivery of resveratrol using solid lipid nanoparticles functionalized with apolipoprotein E. J Nanobiotechnol. 2016;14:27.
Neves AR, Queiroz JF, Weksler B, Romero IA, Couraud PO, Reis S. Solid lipid nanoparticles as a vehicle for brain-targeted drug delivery: two new strategies of functionalization with apolipoprotein E. Nanotechnology. 2015;26:495103.
Neves AR, Queiroz JF, Lima SAC, Reis S. Apo E-functionalization of solid lipid nanoparticles enhances brain drug delivery: uptake mechanism and transport pathways. Bioconjug Chem. 2017;28:995–1004.
Weksler B, Romero IA, Couraud PO. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS. 2013;10:16.
Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J: Official Publication FASEB. 2005;19:1872–4.
Poller B, Gutmann H, Krahenbuhl S, Weksler B, Romero I, Couraud PO, et al. The human brain endothelial cell line hCMEC/D3 as a human blood-brain barrier model for drug transport studies. J Neurochem. 2008;107:1358–68.
Sabateand R, Estelrich J. Stimulatory and inhibitory effects of alkyl bromide surfactants on beta-amyloid fibrillogenesis. Langmuir. 2005;21:6944–9.
Nilsson MR. Techniques to study amyloid fibril formation in vitro. Methods. 2004;34:151–60.
Rocha S, Cardoso I, Borner H, Pereira MC, Saraiva MJ, Coelho M. Design and biological activity of beta-sheet breaker peptide conjugates. Biochem Bioph Res Commun. 2009;380:397–401.
Jameson LP, Smith NW, Dzyuba SV. Dye-binding assays for evaluation of the effects of small molecule inhibitors on amyloid (abeta) self-assembly. ACS Chem Neurosci. 2012;3:807–19.
LeVine H 3rd. Thioflavine T interaction with synthetic Alzheimer's disease beta-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 1993;2:404–10.
Yan Z, Yang Y, Wei X, Zhong J, Wei D, Liu L, et al. Tumor-penetrating peptide mediation: an effective strategy for improving the transport of liposomes in tumor tissue. Mol Pharm. 2014;11:218–25.
Wei X, Zhan C, Chen X, Hou J, Xie C, Lu W. Retro-inverso isomer of Angiopep-2: a stable d-peptide ligand inspires brain-targeted drug delivery. Mol Pharm. 2014;11:3261–8.
Gaumet M, Vargas A, Gurny R, Delie F. Nanoparticles for drug delivery: the need for precision in reporting particle size parameters. Eur J Pharm Biopharm: Official J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2008;69:1–9.
Naseri N, Valizadeh H, Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure. Prep Appl Adv Pharm Bull. 2015;5:305–13.
Freitasand C, Muller RH. Correlation between long-term stability of solid lipid nanoparticles (SLN) and crystallinity of the lipid phase. Eur J Pharm Biopharm: Official J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 1999;47:125–32.
Burdand JF, Usategui-Gomez M. A colorimetric assay for serum lactate dehydrogenase. Clin Chim Acta; Int J Clin Chem. 1973;46:223–7.
Korzeniewskiand C, Callewaert DM. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983;64:313–20.
Phoolcharoen W, Prehaud C, van Dolleweerd CJ, Both L, da Costa A, Lafon M, et al. Enhanced transport of plant-produced rabies single-chain antibody-RVG peptide fusion protein across an in cellulo blood-brain barrier device. Plant Biotechnol J. 2017;15:1331–9.
Loureiro JA, Rocha S, Pereira Mdo C. Charged surfactants induce a non-fibrillar aggregation pathway of amyloid-beta peptide. J Pept Sci: Official Publication Eur Pept Soc. 2013;19:581–7.
Loureiro JA, Gomes B, Fricker G, Cardoso I, Ribeiro CA, Gaiteiro C, et al. Dual ligand immunoliposomes for drug delivery to the brain. Colloid Surface B, Biointerfaces. 2015;134:213–9.
Loureiro JA, Andrade S, Duarte A, Neves AR, Queiroz JF, Nunes C, et al. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s disease. Molecules (Basel, Switzerland). 2017;22:277.
Petkova AT, Ishii Y, Balbach JJ, Antzutkin ON, Leapman RD, Delaglio F, et al. A structural model for Alzheimer’s beta -amyloid fibrils based on experimental constraints from solid state NMR. Proc Natl Acad Sci U S A. 2002;99:16742–7.
Pike CJ, Walencewicz-Wasserman AJ, Kosmoski J, Cribbs DH, Glabe CG, Cotman CW. Structure-activity analyses of beta-amyloid peptides: contributions of the beta 25–35 region to aggregation and neurotoxicity. J Neurochem. 1995;64:253–65.
Torok M, Milton S, Kayed R, Wu P, McIntire T, Glabe CG, et al. Structural and dynamic features of Alzheimer’s Abeta peptide in amyloid fibrils studied by site-directed spin labeling. J Biol Chem. 2002;277:40810–5.
Antzutkin ON, Balbach JJ, Tycko R. Site-specific identification of non-beta-strand conformations in Alzheimer’s beta-amyloid fibrils by solid-state NMR. Biophys J. 2003;84:3326–35.
ACKNOWLEDGMENTS AND DISCLOSURES
This work received financial support from the European Union (FEDER funds) and National Funds (FCT/MEC, Fundação para a Ciência e a Tecnologia and Ministério da Educação e Ciência) under the Partnership Agreement PT2020 UID/QUI/50006/2013 - POCI/01/0145/FEDER/007265. Also from the UID/EQU/00511/2019—Laboratory for Process Engineering, Environment, Biotechnology and Energy—LEPABE, funded by national funds through FCT/MCTES (PIDDAC); Project POCI-01-0145-FEDER-006939, funded by FEDER funds through COMPETE2020—Programa Operacional Competitividade e Internacionalização (POCI) and by national funds (PIDDAC) through FCT/MCTES;—Project “LEPABE-2-ECO-INNOVATION”—NORTE-01-0145-FEDER-000005, funded by Norte Portugal Regional Operational Programme (NORTE 2020), under PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). ARN thanks her previous Post-Doc grant under the project NORTE-01-0145-FEDER-000011. ARN also acknowledges ARDITI for her current Post-Doc grant (ARDITI-CQM_2017_011-PDG) under the project M1420-01-0145-FEDER-000005-CQM+ and the CQM strategic program PEst-OE/QUI/UI0674/2019. Andreia Granja thanks FCT for the PhD grant (SFRH/BD/130147/2017). MP thanks FCT for funding through program DL 57/2016 –Norma transitória.
The authors thank Dr. Mariana Andrade (CEMUP, UP) for technical assistance with NMR experiments and Dr. Rui Fernandes (i3S, UP) for expert help with TEM. We are also thankful to Dr. Babette Weksler from Weill Cornell Medical College (New York, USA), Dr. Ignacio A. Romero from The Open University (Milton Keynes, UK) and Dr. Pierre-Olivier Couraud from INSERM (Paris, France) for technical assistance and support with the hCMEC/D3 cell culture.
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Quercetin-loaded lipid nanoparticles functionalized with RVG29 were developed;
• No cytotoxicity of nanoparticles was detected in hCMEC/D3 cell line;
• RVG29-nanoparticles clearly increased in 1.5-fold the permeability across the BBB;
• The nanoparticles confer protection against amyloid-beta fibrillation;
• Great potential for neuroprotection in Alzheimer’s disease.
Rights and permissions
About this article
Cite this article
Pinheiro, R., Granja, A., Loureiro, J. et al. RVG29-Functionalized Lipid Nanoparticles for Quercetin Brain Delivery and Alzheimer’s Disease. Pharm Res 37, 139 (2020). https://doi.org/10.1007/s11095-020-02865-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02865-1