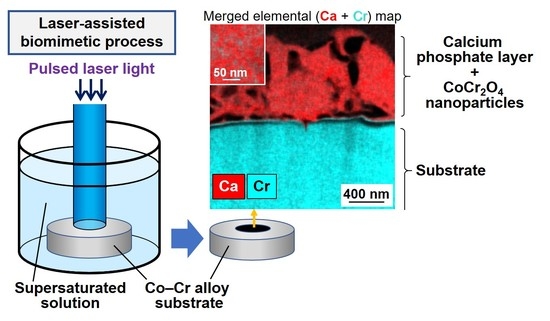
Formation of a Calcium Phosphate Layer with Immobilized Cobalt Chromite Nanoparticles on Cobalt−Chromium Alloy by a Laser-Assisted Biomimetic Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Co−Cr Alloy Substrates
2.2. Preparation of CP Solution
2.3. LAB Process for CaP Coating
2.4. Control Experiments
2.5. Surface Analysis
2.6. Cross-Sectional Analysis
3. Results
3.1. Effects of Irradiation Time
3.2. Effects of Laser Irradiation
3.3. Ultrastructural Analysis of the Surface Layer
3.4. Ultrastructural Analysis of the Substrate Interface
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate bioceramics. Ceram. Int. 2015, 41, 13913–13966. [Google Scholar] [CrossRef]
- Jabbari, Y.S.A. Physico-mechanical properties and prosthodontic applications of Co-Cr dental alloys: A review of the literature. J. Adv. Prosthodont. 2014, 6, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interf. 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [Green Version]
- Grandfield, K.; Palmquist, A.; Gonçalves, S.; Taylor, A.; Taylor, M.; Emanuelsson, L.; Thomsen, P.; Engqvist, H. Free form fabricated features on CoCr implants with and without hydroxyapatite coating in vivo: A comparative study of bone contact and bone growth induction. J. Mater. Sci. Mater. Med. 2011, 22, 899–906. [Google Scholar] [CrossRef]
- Ergun, C.; Doremus, R.H.; Lanford, W.A. Interface reaction/diffusion in hydroxylapatite-coated SS316L and CoCrMo alloys. Acta Mater. 2004, 52, 4767–4772. [Google Scholar] [CrossRef]
- Ortiz, J.C.; Cortés, D.A.; Escobedo, J.C.; Almanza, J.M.; Muñiz, C.R.; Luna, J.S.; Rodríguez, N.A. Bioactive coating on a cobalt base alloy by heat treatment. Mater. Lett. 2011, 65, 329–332. [Google Scholar] [CrossRef]
- Shirdar, M.R.; Izman, S.; Kheimehsari, H.M.; Ahmad, N.; Ma’aram, A. Evaluation of mechanical and electrochemical properties of FHA-coated Co–Cr implant. Surf. Innov. 2017, 5, 90–96. [Google Scholar] [CrossRef]
- Khfagi, O.; Thovhogi, N.; Gihwala, D.; Maaza, M.; Mars, J.A. Synthesis of Co-Cr-Mo fluorapatite nano-composite coatings by pulsed laser deposition for dental applications. Mater. Sci. Appl. 2017, 8, 135–152. [Google Scholar] [CrossRef] [Green Version]
- Redhwi, I.; Lan, T.; Padalkar, S.; Shrotriya, P. Picosecond laser based additive manufacturing of hydroxyapatite coatings on cobalt chromium surfaces. Procedia Manufact. 2018, 26, 125–131. [Google Scholar] [CrossRef]
- Chen, C.; Yao, C.; Yang, J.; Luo, D.; Kong, X.; Chung, S.M.; Lee, I.S. Biomimetic apatite formed on cobalt-chromium alloy: A polymer-free carrier for drug eluting stent. Col. Surf. B Biointerf. 2017, 151, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Surmenev, R.A.; Surmeneva, M.A.; Ivanova, A.A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis? A review. Acta Biomater. 2014, 10, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; de Groot, K.; Wang, D.; Hu, Q.; Wismeijer, D.; Liu, Y. A review paper on biomimetic calcium phosphate coatings. Open Biomed. Eng. J. 2015, 9, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Oyane, A.; Sakamaki, I.; Shimizu, Y.; Kawaguchi, K.; Koshizaki, N. Liquid-phase laser process for simple and area-specific calcium phosphate coating. J. Biomed. Mater. Res. A 2012, 100A, 2573–2580. [Google Scholar] [CrossRef]
- Nakamura, M.; Oyane, A. Physicochemical fabrication of calcium phosphate-based thin layers and nanospheres using laser processing in solutions. J. Mater. Chem. B 2016, 4, 6289–6301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pecheva, E.; Petrov, T.; Lungu, C.; Montgomery, P.; Pramatarova, L. Stimulated in vitro bone-like apatite formation by a novel laser processing technique. Chem. Eng. 2008, 137, 144–153. [Google Scholar] [CrossRef]
- Lee, B.H.; Oyane, A.; Tsurushima, H.; Shimizu, Y.; Sasaki, T.; Koshizaki, N. A new approach for hydroxyapatite coating on polymeric materials using laser-induced precursor formation and subsequent aging. ACS Appl. Mater. Interf. 2009, 1, 1520–1524. [Google Scholar] [CrossRef]
- Oyane, A.; Nakamura, M.; Sakamaki, I.; Shimizu, Y.; Miyata, S.; Miyaji, H. Laser-assisted wet coating of calcium phosphate for surface-functionalization of PEEK. PLoS ONE 2018, 13, e0206524. [Google Scholar] [CrossRef] [Green Version]
- Mahanti, M.; Nakamura, M.; Pyatenko, A.; Sakamaki, I.; Koga, K.; Oyane, A. The mechanism underlying calcium phosphate precipitation on titanium via ultraviolet, visible, and near infrared laser-assisted biomimetic process. J. Phys. D Appl. Phys. 2016, 49, 304003. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Amorphous calcium orthophosphates: Nature, chemistry and biomedical applications. Int. J. Mater. Chem. 2012, 2, 19–46. [Google Scholar] [CrossRef]
- Hanawa, T.; Hiromoto, S.; Asami, K. Characterization of the surface oxide film of a Co–Cr–Mo alloy after being located in quasi-biological environments using XPS. Appl. Surf. Sci. 2001, 183, 68–75. [Google Scholar] [CrossRef]
- McDowell, H.; Gregory, T.M.; Brown, W.E. Solubility of Ca5(PO4)3OH in the system Ca(OH)2–H3PO4–H2O at 5, 15, 25 and 37 °C. J. Res. Natl. Bur. Stand. A Phys. Chem. 1977, 81A, 273–281. [Google Scholar] [CrossRef]
- Van Kemenade, M.J.J.M.; de Bruyn, P.L. A kinetic study of precipitation from supersaturated calcium phosphate solutions. J. Col. Interf. Sci. 1987, 118, 564–585. [Google Scholar] [CrossRef]
- Tung, M.S.; Eidelman, N.; Sieck, B.; Brown, W.E. Octacalcium phosphate solubility product from 4 to 37 °C. J. Res. Natl. Bur. Stand. 1988, 93, 613–624. [Google Scholar] [CrossRef]
- Christoffersen, M.R.; Christoffersen, J.; Kibalczyc, W. Apparent solubilities of two amorphous calcium phosphates and of octacalcium phosphate in the temperature range 30–42 °C. J. Cryst. Growth 1990, 106, 349–354. [Google Scholar] [CrossRef]
- Boom, R.; de Boer, F.R. Energy effects in bulk metals. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–7. [Google Scholar]
- Onuma, K.; Ito, A. Cluster growth model for hydroxyapatite. Chem. Mater. 1998, 10, 3346–3351. [Google Scholar] [CrossRef]
- Dey, A.; Bomans, P.H.H.; Müller, F.A.; Will, J.; Frederik, P.M.; de With, G.; Sommerdijk, N.A.J.M. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nat. Mater. 2010, 9, 1010–1014. [Google Scholar] [CrossRef]
- Zeng, H.; Du, X.W.; Singh, S.C.; Kulinich, S.A.; Yang, S.; He, J.; Cai, W. Nanomaterials via laser ablation/irradiation in liquid: A review. Adv. Funct. Mater. 2012, 22, 1333–1353. [Google Scholar] [CrossRef]
- Burnin, A.; BelBruno, J.J.; Gibson, U.J. Evidence of chromium-cobalt binary cluster formation by pulsed laser evaporation. Inter. J. Mass Spect. 2015, 380, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Habraken, W.J.E.M.; Tao, J.; Brylka, L.J.; Friedrich, H.; Bertinetti, L.; Schenk, A.S.; Verch, A.; Dmitrovic, V.; Bomans, P.H.H.; Frederik, P.M.; et al. Ion-association complexes unite classical and non-classical theories for the biomimetic nucleation of calcium phosphate. Nat. Comm. 2013, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, M.; Oyane, A.; Shimizu, Y.; Miyata, S.; Saeki, A.; Miyaji, H. Physicochemical fabrication of antibacterial calcium phosphate submicrospheres with dispersed silver nanoparticles via coprecipitation and photoreduction under laser irradiation. Acta Biomater. 2016, 46, 299–307. [Google Scholar] [CrossRef]
- Nakamura, M.; Oyane, A.; Sakamaki, I.; Shimizu, Y.; Koga, K.; Koshizaki, N. A physicochemical process for fabricating submicrometer hollow fluorescent spheres of Tb3+-incorporated calcium phosphate. RSC Adv. 2015, 5, 22620–22624. [Google Scholar] [CrossRef]
- Wang, H.; Pyatenko, A.; Kawaguchi, K.; Li, X.; Swiatkowska-Warkocka, Z.; Koshizaki, N. Selective pulsed heating for the synthesis of semiconductor and metal submicrometer spheres. Angew. Chem. Int. Ed. 2010, 49, 6361–6364. [Google Scholar] [CrossRef]
- Li, X.; Shimizu, Y.; Pyatenko, A.; Wang, H.; Koshizaki, N. Carbon-assisted fabrication of submicrometre spheres for low-optical-absorbance materials by selective laser heating in liquid. J. Mater. Chem. 2011, 21, 14406–14409. [Google Scholar] [CrossRef]
- Li, X.; Shimizu, Y.; Pyatenko, A.; Wang, H.; Koshizaki, N. Tetragonal zirconia spheres fabricated by carbon-assisted selective laser heating in a liquid medium. Nanotechnology 2012, 23, 115602. [Google Scholar] [CrossRef]
- Yokota, S.; Nishiwaki, N.; Ueda, K.; Narushima, T.; Kawamura, H.; Takahashi, T. Evaluation of thin amorphous calcium phosphate coatings on titanium dental implants deposited using magnetron sputtering. Implant. Dent. 2014, 23, 343–350. [Google Scholar] [CrossRef]
- Masamoto, K.; Fujibayashi, S.; Yabutsuka, T.; Hiruta, T.; Otsuki, B.; Okuzu, Y.; Goto, K.; Shimizu, T.; Shimizu, Y.; Ishizaki, C.; et al. In vivo and in vitro bioactivity of a ‘‘precursor of apatite” treatment on polyetheretherketone. Acta Biomater. 2019, 91, 48–59. [Google Scholar] [CrossRef]
- Dutta, D.P.; Manjanna, J.; Tyagi, A.K. Magnetic properties of sonochemically synthesized CoCr2O4 nanoparticles. J. Appl. Phys. 2009, 106, 043915. [Google Scholar] [CrossRef]
- Rath, C.; Mohanty, P.; Banerjee, A. Magnetic properties of nanoparticles of cobalt chromite. J. Magn. Magn. Mater. 2011, 323, 1698–1702. [Google Scholar] [CrossRef]
- Gingasu, D.; Mindru, I.; Culita, D.C.; Patron, L.; Calderon-Moreno, J.M.; Osiceanu, P.; Preda, S.; Oprea, O.; Parvulescu, V.; Teodorescu, V.; et al. Structural, magnetic and catalytic properties of cobalt chromite obtained through precursor method. Mater. Res. Bul. 2015, 62, 52–64. [Google Scholar] [CrossRef]
- Fardood, S.T.; Forootan, R.; Moradnia, F.; Afshari, Z.; Ramazani, A. Green synthesis, characterization, and photocatalytic activity of cobalt chromite spinel nanoparticles. Mater. Res. Exp. 2020, 7, 015086. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyane, A.; Sakamaki, I.; Koga, K.; Nakamura, M. Formation of a Calcium Phosphate Layer with Immobilized Cobalt Chromite Nanoparticles on Cobalt−Chromium Alloy by a Laser-Assisted Biomimetic Process. Appl. Sci. 2020, 10, 5584. https://doi.org/10.3390/app10165584
Oyane A, Sakamaki I, Koga K, Nakamura M. Formation of a Calcium Phosphate Layer with Immobilized Cobalt Chromite Nanoparticles on Cobalt−Chromium Alloy by a Laser-Assisted Biomimetic Process. Applied Sciences. 2020; 10(16):5584. https://doi.org/10.3390/app10165584
Chicago/Turabian StyleOyane, Ayako, Ikuko Sakamaki, Kenji Koga, and Maki Nakamura. 2020. "Formation of a Calcium Phosphate Layer with Immobilized Cobalt Chromite Nanoparticles on Cobalt−Chromium Alloy by a Laser-Assisted Biomimetic Process" Applied Sciences 10, no. 16: 5584. https://doi.org/10.3390/app10165584