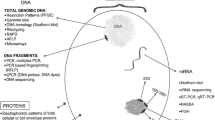
Abstract
Antimicrobial resistance (AMR) is a public health threat where efficient surveillance is needed to prevent outbreaks. Existing methods for detection of gastrointestinal colonization of multidrug-resistant organisms (MDRO) are limited to specific organisms or resistance mechanisms. Metagenomic next-generation sequencing (mNGS) is a more rapid and agnostic diagnostic approach for microbiome and resistome investigations. We determined if mNGS can detect MDRO from rectal swabs in concordance with standard microbiology results. We performed and compared mNGS performance on short-read Illumina MiSeq (N = 10) and long-read Nanopore MinION (N = 5) platforms directly from rectal swabs to detect vancomycin-resistant enterococci (VRE) and carbapenem-resistant Gram-negative organisms (CRO). We detected Enterococcus faecium (N = 8) and Enterococcus faecalis (N = 2) with associated van genes (9/10) in concordance with VRE culture-based results. We studied the microbiome and identified CRO, Pseudomonas aeruginosa (N = 1), Enterobacter cloacae (N = 1), and KPC-producing Klebsiella pneumoniae (N = 1). Nanopore real-time analysis detected the blaKPC gene in 2.3 min and provided genetic context (blaKPC harbored on pKPC_Kp46 IncF plasmid). Illumina sequencing provided accurate allelic variant determination (i.e., blaKPC-2) and strain typing of the K. pneumoniae (ST-15). We demonstrated an agnostic approach for surveillance of MDRO, examining advantages of both short- and long-read mNGS methods for AMR detection.



Similar content being viewed by others
References
Centers for Disease Control and Prevention. (2019) Antibiotic resistance threats in the United States, 2019. CDC
Simner PJ, Martin I, Opene B, Tamma PD, Carroll KC, Milstone AM (2016) Evaluation of multiple methods for detection of gastrointestinal colonization of carbapenem-resistant organisms from rectal swabs. J Clin Microbiol 54(6):1664–1667. https://doi.org/10.1128/jcm.00548-16
Li X, Arias CA, Aitken SL, Galloway Pena J, Panesso D, Chang M, Diaz L, Rios R, Numan Y, Ghaoui S, DebRoy S, Bhatti MM, Simmons DE, Raad I, Hachem R, Folan SA, Sahasarabhojane P, Kalia A, Shelburne SA (2018) Clonal emergence of invasive multidrug-resistant Staphylococcus epidermidis deconvoluted via a combination of whole-genome sequencing and microbiome analyses. Clin Infect Dis. https://doi.org/10.1093/cid/ciy089
Clinical and Laboratory Standards Institute. (2019) Performance standards for antimicrobial susceptibility testing; Twenty-Nineth Informational Supplement., vol M100-S29. CLSI, Wayne, PA, USA.
Tamma PD, Fan Y, Bergman Y, Pertea G, Kazmi AQ, Lewis S, Carroll KC, Schatz MC, Timp W, Simner PJ (2019) Applying rapid whole-genome sequencing to predict phenotypic antimicrobial susceptibility testing results among carbapenem-resistant Klebsiella pneumoniae clinical isolates. Antimicrob Agents Chemother 63(1). https://doi.org/10.1128/aac.01923-18
Wood DE, Salzberg SL (2014) Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol 15(3):R46. https://doi.org/10.1186/gb-2014-15-3-r46
Nurk S, Meleshko D, Korobeynikov A, Pevzner PA (2017) metaSPAdes: a new versatile metagenomic assembler. Genome Res 27(5):824–834. https://doi.org/10.1101/gr.213959.116
Breitwieser FP, Salzberg SL (2019) Pavian: interactive analysis of metagenomics data for microbiome studies and pathogen identification. Bioinformatics. https://doi.org/10.1093/bioinformatics/btz715
Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL (2009) BLAST+: architecture and applications. BMC bioinformatics 10:421. https://doi.org/10.1186/1471-2105-10-421
Core Team R (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Kim JO, Song SA, Yoon EJ, Shin JH, Lee H, Jeong SH, Lee K (2017) Outbreak of KPC-2-producing Enterobacteriaceae caused by clonal dissemination of Klebsiella pneumoniae ST307 carrying an IncX3-type plasmid harboring a truncated Tn4401a. Diagn Microbiol Infect Dis 87(4):343–348. https://doi.org/10.1016/j.diagmicrobio.2016.12.012
Lemon JK, Khil PP, Frank KM, Dekker JP (2017) Rapid Nanopore sequencing of plasmids and resistance gene detection in clinical isolates. J Clin Microbiol 55(12):3530–3543. https://doi.org/10.1128/jcm.01069-17
Mu A, Kwong JC, Isles NS, Goncalves da Silva A, Schultz MB, Ballard SA, Lane CR, Carter GP, Williamson DA, Seemann T, Stinear TP, Howden BP (2019) Reconstruction of the genomes of drug-resistant pathogens for outbreak investigation through metagenomic sequencing. mSphere 4 (1). doi:https://doi.org/10.1128/mSphere.00529-18
Golparian D, Dona V, Sanchez-Buso L, Foerster S, Harris S, Endimiani A, Low N, Unemo M (2018) Antimicrobial resistance prediction and phylogenetic analysis of Neisseria gonorrhoeae isolates using the Oxford Nanopore MinION sequencer. Sci Rep 8(1):17596. https://doi.org/10.1038/s41598-018-35750-4
Kafetzopoulou LE, Efthymiadis K, Lewandowski K, Crook A, Carter D, Osborne J, Aarons E, Hewson R, Hiscox JA, Carroll MW, Vipond R, Pullan ST (2018) Assessment of metagenomic Nanopore and Illumina sequencing for recovering whole genome sequences of chikungunya and dengue viruses directly from clinical samples. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin 23 (50). doi:https://doi.org/10.2807/1560-7917.es.2018.23.50.1800228
Schmidt K, Mwaigwisya S, Crossman LC, Doumith M, Munroe D, Pires C, Khan AM, Woodford N, Saunders NJ, Wain J, O’Grady J, Livermore DM (2017) Identification of bacterial pathogens and antimicrobial resistance directly from clinical urines by Nanopore-based metagenomic sequencing. J Antimicrob Chemother 72(1):104–114. https://doi.org/10.1093/jac/dkw397
Nicholls SM, Quick JC, Tang S, Loman NJ (2019) Ultra-deep, long-read Nanopore sequencing of mock microbial community standards. GigaScience 8(5). https://doi.org/10.1093/gigascience/giz043
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Yee, R., Breitwieser, F.P., Hao, S. et al. Metagenomic next-generation sequencing of rectal swabs for the surveillance of antimicrobial-resistant organisms on the Illumina Miseq and Oxford MinION platforms. Eur J Clin Microbiol Infect Dis 40, 95–102 (2021). https://doi.org/10.1007/s10096-020-03996-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-020-03996-4