Abstract
Purpose
We investigated the ability of baseline 2-deoxy-2-[18F]fluoro-d-glucose PET/CT parameters, acquired before the start of immunotherapy, to predict development of hyperprogressive disease (HPD) in melanoma patients. We also evaluated the diagnostic performances of ratios of baseline and first restaging PET/CT parameters to diagnose HPD without information of the tumor growth kinetic ratio (TGKR) that requires pre-baseline imaging before baseline imaging (3 timepoint imaging).
Procedures
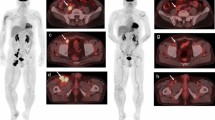
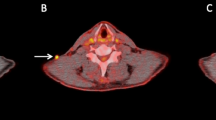
Seventy-six patients who underwent PET/CT before and approximately 3 months following initiation of immunotherapy were included. PET/CT parameters, including metabolic tumor volume (MTV) for all melanoma lesions and total measured tumor burden (TMTB) based on irRECIST, were measured from baseline PET/CT (MTVbase and TMTBbase) and first restaging PET/CT (MTVpost and TMTBpost). The ratios of MTV (MTVpost/MTVbase, MTVr) and TMTB (TMTBpost/TMTBbase, TMTBr) were calculated.
Results
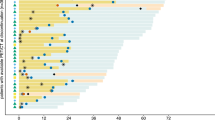
MTVbase of HPD patients (n = 9, TGKR ≥ 2) was larger than that of non-HPD (n = 67, TGKR < 2) patients (P < 0.05), and HPD patients demonstrated shorter median overall survival (7 vs. more than 60 months, P < 0.05). The area under the curve (AUC) of MTVbase (≥ 155.5 ml) to predict the risk of HPD was 0.703, with a sensitivity of 66.7 % and specificity of 81.2 %. The AUCs of MTVr (≥ 1.25) and TMTBr (≥ 1.27) to diagnose HPD without information of TGKR were 0.875 and 0.977 with both sensitivities of 100 %, and specificities of 79 % and 83.9 %, respectively.
Conclusions
Patients at high risk of developing HPD could not be accurately identified based on baseline PET/CT parameters. The ratios of baseline and first restaging PET/CT parameters may be helpful to diagnose HPD, when patients do not undergo pre-baseline imaging.






Similar content being viewed by others
References
Lheureux S, Denoyelle C, Ohashi P, De Bono J, Mottaghy F (2017) Molecularly targeted therapies in cancer: a guide for the nuclear medicine physician. Eur J Nucl Med Mol Imaging 44:41–54
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR (2015) Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med 373:123–135
Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S, Matsumura N, Abiko K, Baba T, Yamaguchi K, Ueda A, Hosoe Y, Morita S, Yokode M, Shimizu A, Honjo T, Konishi I (2015) Safety and antitumor activity of anti–PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol 33:4015–4022
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbé C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372:320–330
Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML (2016) Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 375:1856–1867
Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, le DT, Burtness B, McRee AJ, Lin CC, Pathiraja K, Lunceford J, Emancipator K, Juco J, Koshiji M, Bang YJ (2016) Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 17:717–726
Kurra V, Sullivan RJ, Gainor JF et al (2016) Pseudoprogression in cancer immunotherapy: rates, time course and patient outcomes. Proc Am Soc Clin Oncol
Chiou VL, Burotto M (2015) Pseudoprogression and immune-related response in solid tumors. J Clin Oncol 33:3541–3543
Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, Chaput N, Eggermont A, Marabelle A, Soria JC, Ferté C (2017) Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 23:1920–1928
Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R (2017) Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 23:4242–4250
Saâda-Bouzid E, Defaucheux C, Karabajakian A, Coloma VP, Servois V, Paoletti X, Even C, Fayette J, Guigay J, Loirat D, Peyrade F, Alt M, Gal J, le Tourneau C (2017) Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 28:1605–1611
Tachihara M, Nishimura Y (2019) Who will suffer from hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors. J Thorac Dis 11:S1289–s1291
Fuentes-Antras J, Provencio M, Diaz-Rubio E (2018) Hyperprogression as a distinct outcome after immunotherapy. Cancer Treat Rev 70:16–21
Ito K, Schoder H, Teng R et al (2019) Prognostic value of baseline metabolic tumor volume measured on (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in melanoma patients treated with ipilimumab therapy. Eur J Nucl Med Mol Imaging 46:930–939
Ito K, Teng R, Schoder H et al (2019) (18)F-FDG PET/CT for monitoring of Ipilimumab therapy in patients with metastatic melanoma. J Nucl Med 60:335–341
Son SH, Kang SM, Jeong SY, Lee SW, Lee SJ, Lee J, Ahn BC (2016) Prognostic value of volumetric parameters measured by pretreatment 18F FDG PET/CT in patients with cutaneous malignant melanoma. Clin Nucl Med 41:e266–e273
Bohnsack O, Hoos A, Ludajic K Adaptation of the immune related response criteria: irRECIST [Internet]. OncologyPRO, Lugano, p c2014. [cited 2014 Sep 29]
Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS (2013) Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res 19:3936–3943
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, Mazieres J, Zalcman G, Brosseau S, le Moulec S, Leroy L, Duchemann B, Lefebvre C, Veillon R, Westeel V, Koscielny S, Champiat S, Ferté C, Planchard D, Remon J, Boucher ME, Gazzah A, Adam J, Bria E, Tortora G, Soria JC, Besse B, Caramella C (2018) Hyperprogressive disease in patients with advanced non–small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA oncology 4:1543–1552
Hodi FS, Ballinger M, Lyons B, Soria JC, Nishino M, Tabernero J, Powles T, Smith D, Hoos A, McKenna C, Beyer U, Rhee I, Fine G, Winslow N, Chen DS, Wolchok JD (2018) Immune-modified response evaluation criteria in solid tumors (imRECIST): refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol 36:850–858
Lee JH, Long GV, Menzies AM, Lo S, Guminski A, Whitbourne K, Peranec M, Scolyer R, Kefford RF, Rizos H, Carlino MS (2018) Association between circulating tumor DNA and pseudoprogression in patients with metastatic melanoma treated with anti–programmed cell death 1 antibodies. JAMA Oncol 4:717–721
Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, Hodi FS, Therasse P, Hoekstra OS, Shankar LK, Wolchok JD, Ballinger M, Caramella C, de Vries EGE, RECIST working group (2017) iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 18:e143–e152
Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15:7412–7420
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl 1):122s–150s
Ilyas H, Mikhaeel NG, Dunn JT, Rahman F, Møller H, Smith D, Barrington SF (2018) Defining the optimal method for measuring baseline metabolic tumour volume in diffuse large B cell lymphoma. Eur J Nucl Med Mol Imaging 45:1142–1154
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577
Thompson HM, Minamimoto R, Jamali M, Barkhodari A, von Eyben R, Iagaru A (2016) A prospective, matched comparison study of SUV measurements from time-of-flight versus non–time-of-flight PET/CT scanners. Clin Nucl Med 41:e323–e326
Baratto L, Park SY, Hatami N, Davidzon G, Srinivas S, Gambhir SS, Iagaru A (2017) 18F-FDG silicon photomultiplier PET/CT: a pilot study comparing semi-quantitative measurements with standard PET/CT. PLoS One 12:e0178936
Aide N, Lasnon C, Veit-Haibach P, Sera T, Sattler B, Boellaard R (2017) EANM/EARL harmonization strategies in PET quantification: from daily practice to multicentre oncological studies. Eur J Nucl Med Mol Imaging 44:17–31
Lasnon C, Desmonts C, Quak E, Gervais R, Do P, Dubos-Arvis C, Aide N (2013) Harmonizing SUVs in multicentre trials when using different generation PET systems: prospective validation in non-small cell lung cancer patients. Eur J Nucl Med Mol Imaging 40:985–996
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Institutional Review Board approved this retrospective study and waived the requirement for obtaining written informed consent.
Conflict of Interest
Andrei Iagaru receives institutional research support from GE Healthcare, Advanced Accelerator Applications, and Progenics Pharmaceuticals, unrelated to this work. The other authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nakamoto, R., C Zaba, L., Rosenberg, J. et al. Imaging Characteristics and Diagnostic Performance of 2-deoxy-2-[18F]fluoro-d-Glucose PET/CT for Melanoma Patients Who Demonstrate Hyperprogressive Disease When Treated with Immunotherapy. Mol Imaging Biol 23, 139–147 (2021). https://doi.org/10.1007/s11307-020-01526-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-020-01526-4