Abstract
Background
The mechanical stimulus (i.e., stress or stretch) for growth occurring in the pressure-overloaded left ventricle (LV) is not exactly known.
Objective
To address this issue, we investigate the correlation between local ventricular growth (indexed by local wall thickness) and the local acute changes in mechanical stimuli after aortic banding.
Methods
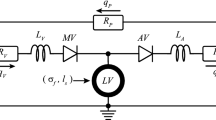
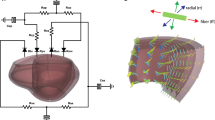
LV geometric data were extracted from 3D echo measurements at baseline and 2 weeks in the aortic banding swine model (n = 4). We developed and calibrated animal-specific finite element (FE) model of LV mechanics against pressure and volume waveforms measured at baseline. After simulation of the acute effects of pressure-overload, the local changes of maximum, mean and minimum myocardial stretches and stresses in three orthogonal material directions (i.e., fiber, sheet and sheet-normal) over a cardiac cycle were quantified. Correlation between mechanical quantities and the corresponding measured local changes in wall thickness was quantified using the Pearson correlation number (PCN) and Spearman rank correlation number (SCN).
Results
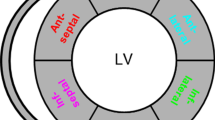
At 2 weeks after banding, the average septum thickness decreased from 10.6 ± 2.92 mm to 9.49 ± 2.02 mm, whereas the LV free-wall thickness increased from 8.69 ± 1.64 mm to 9.4 ± 1.22 mm. The FE results show strong correlation of growth with the changes in maximum fiber stress (PCN = 0.5471, SCN = 0.5111) and changes in the mean sheet-normal stress (PCN = 0.5266, SCN = 0.5256). Myocardial stretches, however, do not have good correlation with growth.
Conclusion
These results suggest that fiber stress is the mechanical stimuli for LV growth in pressure-overload.











Similar content being viewed by others
References
Norton JM (2001) Toward consistent definitions for preload and afterload. Am. J. Physiol. - Adv. Physiol. Educ, TOWARD CONSISTENT DEFINITIONS FOR PRELOAD AND AFTERLOAD
Otto CM (2006) Valvular Aortic Stenosis. In: Valvular Aortic Stenosis. Disease Severity and Timing of Intervention. J. Am. Coll, Cardiol
Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H (2000) Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 343:611–617. https://doi.org/10.1056/NEJM200008313430903
McCrossan ZA, Billeter R, White E (2004) Transmural changes in size, contractile and electrical properties of SHR left ventricular myocytes during compensated hypertrophy. Cardiovasc Res 63:283–292. https://doi.org/10.1016/j.cardiores.2004.04.013
Kichi S, Kawamura K (1991) Architecture of myocardial cells in human cardiac ventricles with concentric and eccentric hypertrophy as demonstrated by quantitative scanning electron microscopy. Heart Vessel 6:129–142. https://doi.org/10.1007/BF02058278
Grossman W, Jones D, McLaurin LP (1975) Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest 56:56–64. https://doi.org/10.1172/JCI108079
Pardo Mindán FJ, Panizo A (1993) Alterations in the extracellular matrix of the myocardium in essential hypertension. Eur Heart J
Carruth ED, McCulloch AD, Omens JH (2016) Transmural gradients of myocardial structure and mechanics: implications for fiber stress and strain in pressure overload. Prog Biophys Mol Biol 122:215–226
Grossman W (1980) Cardiac hypertrophy: useful adaptation or pathologic process? Am J Med 69:576–584
Tamura T, Onodera T, Said S, Gerdes AM (1998) Correlation of myocyte lengthening to chamber dilation in the spontaneously hypertensive heart failure (SHHF) rat. J Mol Cell Cardiol 30:2175–2181. https://doi.org/10.1006/jmcc.1998.0775
Jalil JE, Doering CW, Janicki JS et al (1988) Structural vs. contractile protein remodeling and myocardial stiffness in hypertrophied rat left ventricle. J Mol cell Cardiol. https://doi.org/10.1016/0022-2828(88)90597-4
Yin FC (1981) Ventricular wall stress. Circ Res 49:829–842. https://doi.org/10.1161/01.RES.49.4.829
Shoucri RM (2000) Active and passive stresses in the myocardium. Am J Physiol Heart Circ Physiol 279:H2519–H2528. https://doi.org/10.1152/ajpheart.2000.279.5.h2519
Yang H, Schmidt LP, Wang Z, Yang X, Shao Y, Borg TK, Markwald R, Runyan R, Gao BZ (2016) Dynamic Myofibrillar remodeling in live Cardiomyocytes under static stretch. Sci Rep 6. https://doi.org/10.1038/srep20674
Göktepe S, Abilez OJ, Kuhl E (2010) A generic approach towards finite growth with examples of athlete’s heart, cardiac dilation, and cardiac wall thickening. J Mech Phys Solids 58:1661–1680. https://doi.org/10.1016/j.jmps.2010.07.003
Göktepe S, Abilez OJ, Parker KK, Kuhl E (2010) A multiscale model for eccentric and concentric cardiac growth through sarcomerogenesis. J Theor Biol 265:433–442. https://doi.org/10.1016/j.jtbi.2010.04.023
Rausch MK, Dam A, Göktepe S et al (2011) Computational modeling of growth: systemic and pulmonary hypertension in the heart. Biomech Model Mechanobiol 10:799–811. https://doi.org/10.1007/s10237-010-0275-x
Kerckhoffs RCP, Omens J, McCulloch AD (2012) A single strain-based growth law predicts concentric and eccentric cardiac growth during pressure and volume overload. Mech Res Commun 42:40–50. https://doi.org/10.1016/j.mechrescom.2011.11.004
Yoshida K, McCulloch AD, Omens JH, Holmes JW (2019) Predictions of hypertrophy and its regression in response to pressure overload. Biomech Model Mechanobiol 19:1079–1089. https://doi.org/10.1007/s10237-019-01271-w
Finsberg H, Xi C, Le Tan J et al (2018) Efficient estimation of personalized biventricular mechanical function employing gradient-based optimization. Int j numer method biomed eng:34. https://doi.org/10.1002/cnm.2982
Streeter DD, Spotnitz HM, Patel DP et al (1969) Fiber orientation in the canine left ventricle during diastole and systole. Circ Res 24:339–347. https://doi.org/10.1161/01.RES.24.3.339
Bayer JD, Blake RC, Plank G, Trayanova NA (2012) A novel rule-based algorithm for assigning myocardial fiber orientation to computational heart models. Ann Biomed Eng 40:2243–2254. https://doi.org/10.1007/s10439-012-0593-5
Westerhof N, Lankhaar JW, Westerhof BE (2009) The arterial windkessel. Med Biol Eng Comput 47:131–141
Guccione JM, McCulloch AD, Waldman LK (1991) Passive material properties of intact ventricular myocardium determined from a cylindrical model. J Biomech Eng 113:42–55
Guccione J, Mcculloch A (1993) Mechanics of Actiwe contraction in cardiac muscle : part I — constitutive relations for Fiber stress that describe deactivation. J Biomech Eng:73–81
Guccione JM, Waldman LK, McCulloch AD (1993) Mechanics of Actiwe contraction in cardiac muscle : part II — cylindrical models of the systolic left ventricle. J Biomech Eng 115:82–90
Arumugam J, Mojumder J, Kassab G, Lee LC (2019) Model of anisotropic reverse cardiac growth in mechanical Dyssynchrony. Sci Rep 9:12670. https://doi.org/10.1038/s41598-019-48670-8
Shavik SM, Jiang Z, Baek S, Lee LC (2018) High spatial resolution multi-organ finite element modeling of ventricular-arterial coupling. Front Physiol 9. https://doi.org/10.3389/fphys.2018.00119
Alnæs M, Blechta J, Hake J, et al (2015) The FEniCS Project Version 1.5. Arch Numer Softw 3:. https://doi.org/10.11588/ANS.2015.100.20553
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan TJ, Verani MS (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. J Cardiovasc Magn Reson 4:203–210. https://doi.org/10.1081/JCMR-120003946
Gyöngyösi M, Pavo N, Lukovic D, Zlabinger K, Spannbauer A, Traxler D, Goliasch G, Mandic L, Bergler-Klein J, Gugerell A, Jakab A, Szankai Z, Toth L, Garamvölgyi R, Maurer G, Jaisser F, Zannad F, Thum T, Bátkai S, Winkler J (2017) Porcine model of progressive cardiac hypertrophy and fibrosis with secondary postcapillary pulmonary hypertension. J Transl Med 15:202. https://doi.org/10.1186/s12967-017-1299-0
Sorensen M, Hasenkam JM, Jensen H, Sloth E (2011) Subcoronary versus supracoronary aortic stenosis. An experimental evaluation J Cardiothorac Surg 6. https://doi.org/10.1186/1749-8090-6-100
Aoyagi T, Mirsky I, Flanagan MF et al (1992) Myocardial function in immature and mature sheep with pressure-overload hypertrophy. Am J Phys 262:H1036–H1048
Aoyagi T, Fujii AM, Flanagan MF, Arnold LW, Brathwaite KW, Colan SD, Mirsky I (1993) Transition from compensated hypertrophy to intrinsic myocardial dysfunction during development of left ventricular pressure-overload hypertrophy in conscious sheep: systolic dysfunction precedes diastolic dysfunction. Circulation. 88:2415–2425. https://doi.org/10.1161/01.CIR.88.5.2415
Merino D, Gil A, Gómez J, Ruiz L, Llano M, García R, Hurlé MA, Nistal JF (2018) Experimental modelling of cardiac pressure overload hypertrophy: modified technique for precise, reproducible, safe and easy aortic arch banding-debanding in mice. Sci Rep 8:3167. https://doi.org/10.1038/s41598-018-21548-x
Ishikawa K, Aguero J, Oh JG, Hammoudi N, A. Fish L, Leonardson L, Picatoste B, Santos-Gallego CG, M. Fish K, Hajjar RJ (2015) Increased stiffness is the major early abnormality in a pig model of severe aortic stenosis and predisposes to congestive heart failure in the absence of systolic dysfunction. J Am Heart Assoc:4. https://doi.org/10.1161/JAHA.115.001925
Charles CJ, Lee P, Li RR, Yeung T, Ibraham Mazlan SM, Tay ZW, Abdurrachim D, Teo XQ, Wang WH, Kleijn DPV, Cozzone PJ, Lam CSP, Richards AM (2020) A porcine model of heart failure with preserved ejection fraction: magnetic resonance imaging and metabolic energetics. ESC Hear Fail 7:93–103. https://doi.org/10.1002/ehf2.12536
Lee LC, Kassab GS, Guccione JM (2016) Mathematical modeling of cardiac growth and remodeling. Wiley Interdiscip Rev Syst Biol Med 8:211–226. https://doi.org/10.1002/wsbm.1330
Ambrosi D, Ateshian GA, Arruda EM et al (2011) Perspectives on biological growth and remodeling. J Mech Phys Solids 59:863–883. https://doi.org/10.1016/j.jmps.2010.12.011
Cowin SC (2004) Tissue growth and remodeling. Annu Rev Biomed Eng 6:77–107. https://doi.org/10.1146/annurev.bioeng.6.040803.140250
Maksuti E, Westerhof BE, Ugander M, Donker DW, Carlsson M, Broomé M (2019) Cardiac remodeling in aortic and mitral valve disease: a simulation study with clinical validation. J Appl Physiol 126:1377–1389. https://doi.org/10.1152/japplphysiol.00791.2018
Carasso S, Cohen O, Mutlak D, Adler Z, Lessick J, Reisner SA, Rakowski H, Bolotin G, Agmon Y (2009) Differential effects of afterload on left ventricular long- and short-axis function: insights from a clinical model of patients with aortic valve stenosis undergoing aortic valve replacement. Am Heart J 158:540–545. https://doi.org/10.1016/j.ahj.2009.07.008
Leonardi B, Margossian R, Sanders SP, Chinali M, Colan SD (2014) Ventricular mechanics in patients with aortic valve disease: longitudinal, radial, and circumferential components. Cardiol Young 24:105–112. https://doi.org/10.1017/S1047951112002326
Ozkan A, Kapadia S, Tuzcu M, Marwick TH (2011) Assessment of left ventricular function in aortic stenosis. Nat Rev Cardiol 8:494–501. https://doi.org/10.1038/nrcardio.2011.80
Crozatier B, Caillet D, Bical O (1984) Left ventricular adaptation to sustained pressure overload in the conscious dog. Circ Res 54:21–29. https://doi.org/10.1161/01.RES.54.1.21
McCain ML, Parker KK (2011) Mechanotransduction: the role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflugers Arch 462:89–104. https://doi.org/10.1007/s00424-011-0951-4
Lyon RC, Zanella F, Omens JH, Sheikh F (2015) Mechanotransduction in cardiac hypertrophy and failure. Circ Res 116:1462–1476. https://doi.org/10.1161/CIRCRESAHA.116.304937
Ruwhof C, Van Der Laarse A (2000) Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc Res 47:23–37
Streeter DD, Bassett DL (1966) An engineering analysis of myocardial fiber orientation in pig’s left ventricle in systole. Anat Rec 155(4):503–511. https://doi.org/10.1002/ar.1091550403
Papadacci C, Finel V, Provost J, Villemain O, Bruneval P, Gennisson JL, Tanter M, Fink M, Pernot M (2017) Imaging the dynamics of cardiac fiber orientation in vivo using 3D ultrasound backscatter tensor imaging. Sci Rep 7(1):830. https://doi.org/10.1038/s41598-017-00946-7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This work is supported by National Institutes of Health grants (R01 HL134841 and U01 HL133359) and AHA SDG (17SDG33370110) grant as well as Singapore National Medical Research Council grants (NMRC/BnB/0017/2015; NMRC/OFIRG/0018/2016; MOH-000358). The authors declare that they have no conflicts of interest. All applicable international, national and institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix A (Mesh Sensitivity Analysis)
Calibrated Model Parameters
Model Prediction and Correlation Coefficients of Each Pig Model
Sensitivity Analysis of Changes in Fiber Angles
Rights and permissions
About this article
Cite this article
Mojumder, J., Choy, J.S., Leng, S. et al. Mechanical Stimuli for Left Ventricular Growth During Pressure Overload. Exp Mech 61, 131–146 (2021). https://doi.org/10.1007/s11340-020-00643-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11340-020-00643-z