Abstract
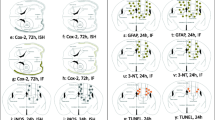
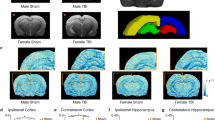
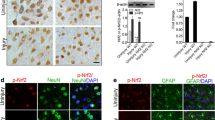
Neurotrauma especially traumatic brain injury (TBI) is the leading cause of death and disability worldwide. To improve upon the early diagnosis and develop precision-targeted therapies for TBI, it is critical to understand the underlying molecular mechanisms and signaling pathways. The transcription factor, nuclear factor kappa B (NFκB), which is ubiquitously expressed, plays a crucial role in the normal cell survival, proliferation, differentiation, function, as well as in disease states like neuroinflammation and neurodegeneration. Here, we hypothesized that real-time noninvasive bioluminescence molecular imaging allows rapid and precise monitoring of TBI-induced immediate and rapid spatio-temporal activation of NFκB signaling pathway in response to Glia maturation factor (GMF) upregulation which in turn leads to neuroinflammation and neurodegeneration post-TBI. To test and validate our hypothesis and to gain novel mechanistic insights, we subjected NFκB-RE-Luc transgenic male and female mice to TBI and performed real-time noninvasive bioluminescence imaging (BLI) as well as photoacoustic and ultrasound imaging (PAI). Our BLI data revealed that TBI leads to an immediate and sustained activation of NFκB signaling. Further, our BLI data suggest that especially in male NFκB-RE-Luc transgenic mice subjected to TBI, in addition to brain, there is widespread activation of NFκB signaling in multiple organs. However, in the case of the female NFκB-RE-Luc transgenic mice, TBI induces a very specific and localized activation of NFκB signaling in the brain. Further, our microRNA data suggest that TBI induces significant upregulation of mir-9-5p, mir-21a-5p, mir-34a-5p, mir-16-3p, as well as mir-155-5p within 24 h and these microRNAs can be successfully used as TBI-specific biomarkers. To the best of our knowledge, this is one of the first and unique study of its kind to report immediate and sustained activation of NFκB signaling post-TBI in a gender-specific manner by utilizing real-time non-invasive BLI and PAI in NFκB-RE-Luc transgenic mice. Our study will prove immensely beneficial to gain novel mechanistic insights underlying TBI, unravel novel therapeutic targets, as well as enable us to monitor in real-time the response to innovative TBI-specific precision-targeted gene and stem cell-based precision medicine.











Similar content being viewed by others
References
Acaz-Fonseca E, Duran JC, Carrero P, Garcia-Segura LM, Arevalo MA (2015) Sex differences in glia reactivity after cortical brain injury. Glia 63(11):1966–1981. https://doi.org/10.1002/glia.22867
Aelvoet SA, Ibrahimi A, Macchi F, Gijsbers R, Van den Haute C, Debyser Z, Baekelandt V (2014) Noninvasive bioluminescence imaging of alpha-synuclein oligomerization in mouse brain using split firefly luciferase reporters. J Neurosci 34(49):16518–16532. https://doi.org/10.1523/JNEUROSCI.4933-13.2014
Ahmed ME, Iyer S, Thangavel R, Kempuraj D, Selvakumar GP, Raikwar SP, Zaheer S, Zaheer A (2017) Co-localization of glia maturation factor with NLRP3 inflammasome and autophagosome markers in human Alzheimer's disease brain. J Alzheimers Dis 60(3):1143–1160. https://doi.org/10.3233/JAD-170634
Ahmed ME, Selvakumar GP, Kempuraj D, Raikwar SP, Thangavel R, Bazley K, Wu K, Khan O, Kukulka K, Bussinger B, Dubova I, Zaheer S, Govindarajan R, Iyer S, Burton C, James D, Zaheer A (2020a) Neuroinflammation mediated by glia maturation factor exacerbates neuronal injury in an in vitro model of traumatic brain injury. J Neurotrauma. https://doi.org/10.1089/neu.2019.6932
Ahmed ME, Selvakumar GP, Thangavel R, Kempuraj D, Raikwar SP, Zaheer S, Iyer S, Zaheer A (2020b) Immune suppression of glia maturation factor reverses behavioral impairment, attenuates amyloid plaque pathology and neuroinflammation in an Alzheimer's disease mouse model. J Neuroimmune Pharmacol. https://doi.org/10.1007/s11481-020-09929-4
Akimoto H, Kwon HJ, Ozaki M, Yasuda K, Honma K, Ohmiya Y (2009) In vivo bioluminescence imaging of bone marrow-derived cells in brain inflammation. Biochem Biophys Res Commun 380(4):844–849. https://doi.org/10.1016/j.bbrc.2009.01.181
Aswendt M, Vogel S, Schafer C, Jathoul A, Pule M, Hoehn M (2019) Quantitative in vivo dual-color bioluminescence imaging in the mouse brain. Neurophotonics 6(2):025006. https://doi.org/10.1117/1.NPh.6.2.025006
Atif H, Hicks SD (2019) A review of MicroRNA biomarkers in traumatic brain injury. J Exp Neurosci 13:1179069519832286. https://doi.org/10.1177/1179069519832286
Bruce-Keller AJ, Dimayuga FO, Reed JL, Wang C, Angers R, Wilson ME, Dimayuga VM, Scheff SW (2007) Gender and estrogen manipulation do not affect traumatic brain injury in mice. J Neurotrauma 24(1):203–215. https://doi.org/10.1089/neu.2006.0163
Buckley SM, Delhove JM, Perocheau DP, Karda R, Rahim AA, Howe SJ, Ward NJ, Birrell MA, Belvisi MG, Arbuthnot P, Johnson MR, Waddington SN, McKay TR (2015) In vivo bioimaging with tissue-specific transcription factor activated luciferase reporters. Sci Rep 5:11842. https://doi.org/10.1038/srep11842
Carlsen H, Moskaug JO, Fromm SH, Blomhoff R (2002) In vivo imaging of NF-kappa B activity. J Immunol 168(3):1441–1446. https://doi.org/10.4049/jimmunol.168.3.1441
Clevenger AC, Kim H, Salcedo E, Yonchek JC, Rodgers KM, Orfila JE, Dietz RM, Quillinan N, Traystman RJ, Herson PS (2018) Endogenous sex steroids dampen neuroinflammation and improve outcome of traumatic brain injury in mice. J Mol Neurosci 64(3):410–420. https://doi.org/10.1007/s12031-018-1038-x
Cordeau P, Kriz J (2012) Real-time imaging after cerebral ischemia: model systems for visualization of inflammation and neuronal repair. Methods Enzymol 506:117–133. https://doi.org/10.1016/B978-0-12-391856-7.00031-7
Cordeau P Jr, Lalancette-Hebert M, Weng YC, Kriz J (2008) Live imaging of neuroinflammation reveals sex and estrogen effects on astrocyte response to ischemic injury. Stroke 39(3):935–942. https://doi.org/10.1161/STROKEAHA.107.501460
Cui GH, Wu J, Mou FF, Xie WH, Wang FB, Wang QL, Fang J, Xu YW, Dong YR, Liu JR, Guo HD (2018) Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J 32(2):654–668. https://doi.org/10.1096/fj.201700600R
de Freitas Cardoso MG, Faleiro RM, de Paula JJ, Kummer A, Caramelli P, Teixeira AL, de Souza LC, Miranda AS (2019) Cognitive impairment following acute mild traumatic brain injury. Front Neurol 10:198. https://doi.org/10.3389/fneur.2019.00198
Di Pietro V, Ragusa M, Davies D, Su Z, Hazeldine J, Lazzarino G, Hill LJ, Crombie N, Foster M, Purrello M, Logan A, Belli A (2017) MicroRNAs as novel biomarkers for the diagnosis and prognosis of mild and severe traumatic brain injury. J Neurotrauma 34(11):1948–1956. https://doi.org/10.1089/neu.2016.4857
Di Pietro V, Yakoub KM, Scarpa U, Di Pietro C, Belli A (2018) MicroRNA signature of traumatic brain injury: from the biomarker discovery to the point-of-care. Front Neurol 9:429. https://doi.org/10.3389/fneur.2018.00429
DoD Worldwide Numbers for TBI (2018) Defense and Veterans Brain Injury Center
Dohlen G, Carlsen H, Blomhoff R, Thaulow E, Saugstad OD (2005) Reoxygenation of hypoxic mice with 100% oxygen induces brain nuclear factor-kappa B. Pediatr Res 58(5):941–945. https://doi.org/10.1203/01.PDR.0000182595.62545.EE
Donat CK, Scott G, Gentleman SM, Sastre M (2017) Microglial activation in traumatic brain injury. Front Aging Neurosci 9:208. https://doi.org/10.3389/fnagi.2017.00208
Doran SJ, Ritzel RM, Glaser EP, Henry RJ, Faden AI, Loane DJ (2019) Sex Differences in acute neuroinflammation after experimental traumatic brain injury are mediated by infiltrating myeloid cells. J Neurotrauma 36(7):1040–1053. https://doi.org/10.1089/neu.2018.6019
Fluiter K, Opperhuizen AL, Morgan BP, Baas F, Ramaglia V (2014) Inhibition of the membrane attack complex of the complement system reduces secondary neuroaxonal loss and promotes neurologic recovery after traumatic brain injury in mice. J Immunol 192(5):2339–2348. https://doi.org/10.4049/jimmunol.1302793
Fricke IB, Schelhaas S, Zinnhardt B, Viel T, Hermann S, Couillard-Despres S, Jacobs AH (2017) In vivo bioluminescence imaging of neurogenesis—the role of the blood brain barrier in an experimental model of Parkinson's disease. Eur J Neurosci 45(7):975–986. https://doi.org/10.1111/ejn.13540
Fukuchi M, Izumi H, Mori H, Kiyama M, Otsuka S, Maki S, Maehata Y, Tabuchi A, Tsuda M (2017) Visualizing changes in brain-derived neurotrophic factor (BDNF) expression using bioluminescence imaging in living mice. Sci Rep 7(1):4949. https://doi.org/10.1038/s41598-017-05297-x
Gao X, Chen J (2011) Mild traumatic brain injury results in extensive neuronal degeneration in the cerebral cortex. J Neuropathol Exp Neurol 70(3):183–191. https://doi.org/10.1097/NEN.0b013e31820c6878
Ge XT, Lei P, Wang HC, Zhang AL, Han ZL, Chen X, Li SH, Jiang RC, Kang CS, Zhang JN (2014) miR-21 improves the neurological outcome after traumatic brain injury in rats. Sci Rep 4:6718. https://doi.org/10.1038/srep06718
Ge X, Han Z, Chen F, Wang H, Zhang B, Jiang R, Lei P, Zhang J (2015) MiR-21 alleviates secondary blood-brain barrier damage after traumatic brain injury in rats. Brain Res 1603:150–157. https://doi.org/10.1016/j.brainres.2015.01.009
Giustetto P, Filippi M, Castano M, Terreno E (2015) Non-invasive parenchymal, vascular and metabolic high-frequency ultrasound and photoacoustic rat deep brain imaging. J Vis Exp 97:e52162. https://doi.org/10.3791/52162
Hall ED, Gibson TR, Pavel KM (2005) Lack of a gender difference in post-traumatic neurodegeneration in the mouse controlled cortical impact injury model. J Neurotrauma 22(6):669–679. https://doi.org/10.1089/neu.2005.22.669
Harrison EB, Hochfelder CG, Lamberty BG, Meays BM, Morsey BM, Kelso ML, Fox HS, Yelamanchili SV (2016) Traumatic brain injury increases levels of miR-21 in extracellular vesicles: implications for neuroinflammation. FEBS Open Bio 6(8):835–846. https://doi.org/10.1002/2211-5463.12092
Hicks SD, Johnson J, Carney MC, Bramley H, Olympia RP, Loeffert AC, Thomas NJ (2018) Overlapping MicroRNA expression in saliva and cerebrospinal fluid accurately identifies pediatric traumatic brain injury. J Neurotrauma 35(1):64–72. https://doi.org/10.1089/neu.2017.5111
Hochgrafe K, Mandelkow EM (2013) Making the brain glow: in vivo bioluminescence imaging to study neurodegeneration. Mol Neurobiol 47(3):868–882. https://doi.org/10.1007/s12035-012-8379-1
Hoey C, Ahmed M, Fotouhi Ghiam A, Vesprini D, Huang X, Commisso K, Commisso A, Ray J, Fokas E, Loblaw DA, He HH, Liu SK (2019) Circulating miRNAs as non-invasive biomarkers to predict aggressive prostate cancer after radical prostatectomy. J Transl Med 17(1):173. https://doi.org/10.1186/s12967-019-1920-5
Hu YC, Sun Q, Li W, Zhang DD, Ma B, Li S, Li WD, Zhou ML, Hang CH (2014) Biphasic activation of nuclear factor kappa B and expression of p65 and c-Rel after traumatic brain injury in rats. Inflamm Res 63(2):109–115. https://doi.org/10.1007/s00011-013-0677-1
Hu T, Zhou FJ, Chang YF, Li YS, Liu GC, Hong Y, Chen HL, Xiyang YB, Bao TH (2015) miR21 is associated with the cognitive improvement following voluntary running wheel exercise in TBI mice. J Mol Neurosci 57(1):114–122. https://doi.org/10.1007/s12031-015-0584-8
Iwano S, Sugiyama M, Hama H, Watakabe A, Hasegawa N, Kuchimaru T, Tanaka KZ, Takahashi M, Ishida Y, Hata J, Shimozono S, Namiki K, Fukano T, Kiyama M, Okano H, Kizaka-Kondoh S, McHugh TJ, Yamamori T, Hioki H, Maki S, Miyawaki A (2018) Single-cell bioluminescence imaging of deep tissue in freely moving animals. Science 359(6378):935–939. https://doi.org/10.1126/science.aaq1067
Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J (2017) Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron 95(6):1246–1265. https://doi.org/10.1016/j.neuron.2017.07.010
Kempuraj D, Thangavel R, Natteru PA, Selvakumar GP, Saeed D, Zahoor H, Zaheer S, Iyer SS, Zaheer A (2016) Neuroinflammation induces neurodegeneration. J Neurol Neurosurg Spine 1(1):1003
Klose AD, Paragas N (2018) Automated quantification of bioluminescence images. Nat Commun 9(1):4262. https://doi.org/10.1038/s41467-018-06288-w
Kuchimaru T, Iwano S, Kiyama M, Mitsumata S, Kadonosono T, Niwa H, Maki S, Kizaka-Kondoh S (2016) A luciferin analogue generating near-infrared bioluminescence achieves highly sensitive deep-tissue imaging. Nat Commun 7:11856. https://doi.org/10.1038/ncomms11856
Langlois JA, Rutland-Brown W, Wald MM (2006) The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 21(5):375–378. https://doi.org/10.1097/00001199-200609000-00001
Lei P, Li Y, Chen X, Yang S, Zhang J (2009) Microarray based analysis of microRNA expression in rat cerebral cortex after traumatic brain injury. Brain Res 1284:191–201. https://doi.org/10.1016/j.brainres.2009.05.074
Lim R, Turriff DE, Troy SS, Moore BW, Eng LF (1977) Glia maturation factor: effect on chemical differentiation of glioblasts in culture. Science 195(4274):195–196
Lim R, Nakagawa S, Arnason BG, Turriff DE (1981) Glia maturation factor promotes contact inhibition in cancer cells. Proc Natl Acad Sci USA 78(7):4373–4377
Lim R, Miller JF, Zaheer A (1989) Purification and characterization of glia maturation factor beta: a growth regulator for neurons and glia. Proc Natl Acad Sci USA 86(10):3901–3905
Lipponen A, Paananen J, Puhakka N, Pitkanen A (2016) Analysis of post-traumatic brain injury gene expression signature reveals tubulins, Nfe2l2, Nfkb, Cd44, and S100a4 as treatment targets. Sci Rep 6:31570. https://doi.org/10.1038/srep31570
Lopez de Heredia L, Gengatharan A, Foster J, Mather S, Magoulas C (2011) Bioluminescence imaging of the brain response to acute inflammation in living C/EBP reporter mice. Neurosci Lett 497(2):134–138. https://doi.org/10.1016/j.neulet.2011.04.046
Lucke-Wold B (2018) Understanding the link between traumatic brain injury and Alzheimer's disease. Ann Transl Med 6(Suppl 1):S70. https://doi.org/10.21037/atm.2018.10.42
Lucke-Wold BP, Turner RC, Logsdon AF, Bailes JE, Huber JD, Rosen CL (2014) Linking traumatic brain injury to chronic traumatic encephalopathy: identification of potential mechanisms leading to neurofibrillary tangle development. J Neurotrauma 31(13):1129–1138. https://doi.org/10.1089/neu.2013.3303
Luo J, Nguyen A, Villeda S, Zhang H, Ding Z, Lindsey D, Bieri G, Castellano JM, Beaupre GS, Wyss-Coray T (2014) Long-term cognitive impairments and pathological alterations in a mouse model of repetitive mild traumatic brain injury. Front Neurol 5:12. https://doi.org/10.3389/fneur.2014.00012
Maas AI, Stocchetti N, Bullock R (2008) Moderate and severe traumatic brain injury in adults. Lancet Neurol 7(8):728–741. https://doi.org/10.1016/S1474-4422(08)70164-9
Marklund N, Hillered L (2011) Animal modelling of traumatic brain injury in preclinical drug development: where do we go from here? Br J Pharmacol 164(4):1207–1229. https://doi.org/10.1111/j.1476-5381.2010.01163.x
Marklund N, Bakshi A, Castelbuono DJ, Conte V, McIntosh TK (2006) Evaluation of pharmacological treatment strategies in traumatic brain injury. Curr Pharm Des 12(13):1645–1680. https://doi.org/10.2174/138161206776843340
Morlacchi S, Dal Secco V, Soldani C, Glaichenhaus N, Viola A, Sarukhan A (2011) Regulatory T cells target chemokine secretion by dendritic cells independently of their capacity to regulate T cell proliferation. J Immunol 186(12):6807–6814. https://doi.org/10.4049/jimmunol.1003265
Mukherjee S, Arisi GM, Mims K, Hollingsworth G, O'Neil K, Shapiro LA (2020) Neuroinflammatory mechanisms of post-traumatic epilepsy. J Neuroinflamm 17(1):193. https://doi.org/10.1186/s12974-020-01854-w
Nguyen R, Fiest KM, McChesney J, Kwon CS, Jette N, Frolkis AD, Atta C, Mah S, Dhaliwal H, Reid A, Pringsheim T, Dykeman J, Gallagher C (2016) The international incidence of traumatic brain injury: a systematic review and meta-analysis. Can J Neurol Sci 43(6):774–785. https://doi.org/10.1017/cjn.2016.290
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308(5726):1314–1318. https://doi.org/10.1126/science.1110647
Nonaka M, Chen XH, Pierce JE, Leoni MJ, McIntosh TK, Wolf JA, Smith DH (1999) Prolonged activation of NF-kappaB following traumatic brain injury in rats. J Neurotrauma 16(11):1023–1034. https://doi.org/10.1089/neu.1999.16.1023
Ouellet MC, Beaulieu-Bonneau S, Morin CM (2015) Sleep-wake disturbances after traumatic brain injury. Lancet Neurol 14(7):746–757. https://doi.org/10.1016/S1474-4422(15)00068-X
Pan YB, Sun ZL, Feng DF (2017) The role of MicroRNA in traumatic brain injury. Neuroscience 367:189–199. https://doi.org/10.1016/j.neuroscience.2017.10.046
Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT (2011) Synaptic pruning by microglia is necessary for normal brain development. Science 333(6048):1456–1458. https://doi.org/10.1126/science.1202529
Partridge J, Carlsen H, Enesa K, Chaudhury H, Zakkar M, Luong L, Kinderlerer A, Johns M, Blomhoff R, Mason JC, Haskard DO, Evans PC (2007) Laminar shear stress acts as a switch to regulate divergent functions of NF-kappaB in endothelial cells. FASEB J 21(13):3553–3561. https://doi.org/10.1096/fj.06-8059com
Raikwar SP, Thangavel R, Dubova I, Selvakumar GP, Ahmed ME, Kempuraj D, Zaheer SA, Iyer SS, Zaheer A (2019) Targeted gene editing of glia maturation factor in microglia: a novel Alzheimer's disease therapeutic target. Mol Neurobiol 56(1):378–393. https://doi.org/10.1007/s12035-018-1068-y
Ramos-Cejudo J, Wisniewski T, Marmar C, Zetterberg H, Blennow K, de Leon MJ, Fossati S (2018) Traumatic brain injury and Alzheimer's disease: the cerebrovascular link. EBioMedicine 28:21–30. https://doi.org/10.1016/j.ebiom.2018.01.021
Redell JB, Moore AN, Ward NH 3rd, Hergenroeder GW, Dash PK (2010) Human traumatic brain injury alters plasma microRNA levels. J Neurotrauma 27(12):2147–2156. https://doi.org/10.1089/neu.2010.1481
Report to Congress on Traumatic Brain Injury in the United States: Understanding the Public Health Problem among Current and Former Military Personnel (2013) National Institutes of Health, Centers for Disease Control and Prevention
Rogall R, Rabenstein M, Vay S, Bach A, Pikhovych A, Baermann J, Hoehn M, Couillard-Despres S, Fink GR, Schroeter M, Rueger MA (2018) Bioluminescence imaging visualizes osteopontin-induced neurogenesis and neuroblast migration in the mouse brain after stroke. Stem Cell Res Ther 9(1):182. https://doi.org/10.1186/s13287-018-0927-9
Roof RL, Hall ED (2000) Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma 17(5):367–388. https://doi.org/10.1089/neu.2000.17.367
Roozenbeek B, Maas AI, Menon DK (2013) Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol 9(4):231–236. https://doi.org/10.1038/nrneurol.2013.22
Roth DJ, Jansen ED, Powers AC, Wang TG (2006) A novel method of monitoring response to islet transplantation: bioluminescent imaging of an NF-kB transgenic mouse model. Transplantation 81(8):1185–1190. https://doi.org/10.1097/01.tp.0000203808.84963.13
Sandhir R, Gregory E, Berman NE (2014) Differential response of miRNA-21 and its targets after traumatic brain injury in aging mice. Neurochem Int 78:117–121. https://doi.org/10.1016/j.neuint.2014.09.009
Sato M, Chang E, Igarashi T, Noble LJ (2001) Neuronal injury and loss after traumatic brain injury: time course and regional variability. Brain Res 917(1):45–54. https://doi.org/10.1016/s0006-8993(01)02905-5
Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B (2012) Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74(4):691–705. https://doi.org/10.1016/j.neuron.2012.03.026
Selvakumar GP, Ahmed ME, Iyer SS, Thangavel R, Kempuraj D, Raikwar SP, Bazley K, Wu K, Khan A, Kukulka K, Bussinger B, Zaheer S, Burton C, James D, Zaheer A (2020) Absence of glia maturation factor protects from axonal injury and motor behavioral impairments after traumatic brain injury. Exp Neurobiol. https://doi.org/10.5607/en20017
Sharma A, Chandran R, Barry ES, Bhomia M, Hutchison MA, Balakathiresan NS, Grunberg NE, Maheshwari RK (2014) Identification of serum microRNA signatures for diagnosis of mild traumatic brain injury in a closed head injury model. PLoS ONE 9(11):e112019. https://doi.org/10.1371/journal.pone.0112019
Silverberg ND, Duhaime AC, Iaccarino MA (2019) Mild Traumatic Brain Injury in 2019–2020. JAMA. https://doi.org/10.1001/jama.2019.18134
Smith DH, Johnson VE, Stewart W (2013) Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol 9(4):211–221. https://doi.org/10.1038/nrneurol.2013.29
Surveillance Report of Traumatic Brain Injury-related Emergency Department Visits, Hospitalizations, and Deaths (2014) Centers for Disease Control and Prevention
Susarla BT, Villapol S, Yi JH, Geller HM, Symes AJ (2014) Temporal patterns of cortical proliferation of glial cell populations after traumatic brain injury in mice. ASN Neuro 6(3):159–170. https://doi.org/10.1042/AN20130034
Taheri S, Tanriverdi F, Zararsiz G, Elbuken G, Ulutabanca H, Karaca Z, Selcuklu A, Unluhizarci K, Tanriverdi K, Kelestimur F (2016) Circulating MicroRNAs as potential biomarkers for traumatic brain injury-induced hypopituitarism. J Neurotrauma 33(20):1818–1825. https://doi.org/10.1089/neu.2015.4281
Taylor BC, Hagel Campbell E, Nugent S, Bidelspach DE, Kehle-Forbes SM, Scholten J, Stroupe KT, Sayer NA (2017) Three year trends in veterans health administration utilization and costs after traumatic brain injury screening among veterans with mild traumatic brain injury. J Neurotrauma 34(17):2567–2574. https://doi.org/10.1089/neu.2016.4910
Tennstaedt A, Aswendt M, Adamczak J, Hoehn M (2013) Noninvasive multimodal imaging of stem cell transplants in the brain using bioluminescence imaging and magnetic resonance imaging. Methods Mol Biol 1052:153–166. https://doi.org/10.1007/7651_2013_14
Thangavel R, Stolmeier D, Yang X, Anantharam P, Zaheer A (2012) Expression of glia maturation factor in neuropathological lesions of Alzheimer's disease. Neuropathol Appl Neurobiol 38(6):572–581. https://doi.org/10.1111/j.1365-2990.2011.01232.x
Thangavel R, Kempuraj D, Stolmeier D, Anantharam P, Khan M, Zaheer A (2013) Glia maturation factor expression in entorhinal cortex of Alzheimer's disease brain. Neurochem Res 38(9):1777–1784. https://doi.org/10.1007/s11064-013-1080-6
Thangavel R, Kempuraj D, Zaheer S, Raikwar S, Ahmed ME, Selvakumar GP, Iyer SS, Zaheer A (2017) Glia maturation factor and mitochondrial uncoupling proteins 2 and 4 expression in the temporal cortex of Alzheimer's disease brain. Front Aging Neurosci 9:150. https://doi.org/10.3389/fnagi.2017.00150
Thangavel R, Bhagavan SM, Ramaswamy SB, Surpur S, Govindarajan R, Kempuraj D, Zaheer S, Raikwar S, Ahmed ME, Selvakumar GP, Iyer SS, Zaheer A (2018) Co-expression of glia maturation factor and apolipoprotein E4 in Alzheimer's disease brain. J Alzheimers Dis 61(2):553–560. https://doi.org/10.3233/JAD-170777
Toffolo K, Osei J, Kelly W, Poulsen A, Donahue K, Wang J, Hunter M, Bard J, Wang J, Poulsen D (2019) Circulating microRNAs as biomarkers in traumatic brain injury. Neuropharmacology 145(Pt B):199–208. https://doi.org/10.1016/j.neuropharm.2018.08.028
Upputuri PK, Pramanik M (2019) Photoacoustic imaging in the second near-infrared window: a review. J Biomed Opt 24(4):1–20. https://doi.org/10.1117/1.JBO.24.4.040901
Vasterling JJ, Jacob SN, Rasmusson A (2018) Traumatic brain injury and posttraumatic stress disorder: conceptual, diagnostic, and therapeutic considerations in the context of co-occurrence. J Neuropsychiatry Clin Neurosci 30(2):91–100. https://doi.org/10.1176/appi.neuropsych.17090180
Villapol S, Byrnes KR, Symes AJ (2014) Temporal dynamics of cerebral blood flow, cortical damage, apoptosis, astrocyte-vasculature interaction and astrogliosis in the pericontusional region after traumatic brain injury. Front Neurol 5:82. https://doi.org/10.3389/fneur.2014.00082
Villapol S, Loane DJ, Burns MP (2017) Sexual dimorphism in the inflammatory response to traumatic brain injury. Glia 65(9):1423–1438. https://doi.org/10.1002/glia.23171
Wang WX, Visavadiya NP, Pandya JD, Nelson PT, Sullivan PG, Springer JE (2015) Mitochondria-associated microRNAs in rat hippocampus following traumatic brain injury. Exp Neurol 265:84–93. https://doi.org/10.1016/j.expneurol.2014.12.018
White DL, Kunik ME, Yu H, Lin HL, Richardson PA, Moore S, Sarwar AI, Marsh L, Jorge RE (2020) Post-traumatic stress disorder is associated with further increased Parkinson's disease risk in veterans with traumatic brain injury. Ann Neurol 88(1):33–41. https://doi.org/10.1002/ana.25726
Wu J, He J, Tian X, Li H, Wen Y, Shao Q, Cheng C, Wang G, Sun X (2020) Upregulation of miRNA-9-5p promotes angiogenesis after traumatic Brain injury by inhibiting Ptch-1. Neuroscience 440:160–174. https://doi.org/10.1016/j.neuroscience.2020.05.045
Xiong Y, Mahmood A, Chopp M (2013) Animal models of traumatic brain injury. Nat Rev Neurosci 14(2):128–142. https://doi.org/10.1038/nrn3407
Yin Z, Han Z, Hu T, Zhang S, Ge X, Huang S, Wang L, Yu J, Li W, Wang Y, Li D, Zhao J, Wang Y, Zuo Y, Li Y, Kong X, Chen F, Lei P (2020) Neuron-derived exosomes with high miR-21-5p expression promoted polarization of M1 microglia in culture. Brain Behav Immun 83:270–282. https://doi.org/10.1016/j.bbi.2019.11.004
Zaheer A, Yorek MA, Lim R (2001) Effects of glia maturation factor overexpression in primary astrocytes on MAP kinase activation, transcription factor activation, and neurotrophin secretion. Neurochem Res 26(12):1293–1299
Zaheer A, Mathur SN, Lim R (2002) Overexpression of glia maturation factor in astrocytes leads to immune activation of microglia through secretion of granulocyte-macrophage-colony stimulating factor. Biochem Biophys Res Commun 294(2):238–244. https://doi.org/10.1016/S0006-291X(02)00467-9
Zaheer A, Zaheer S, Sahu SK, Knight S, Khosravi H, Mathur SN, Lim R (2007) A novel role of glia maturation factor: induction of granulocyte-macrophage colony-stimulating factor and pro-inflammatory cytokines. J Neurochem 101(2):364–376. https://doi.org/10.1111/j.1471-4159.2006.04385.x
Zaheer A, Zaheer S, Thangavel R, Wu Y, Sahu SK, Yang B (2008) Glia maturation factor modulates beta-amyloid-induced glial activation, inflammatory cytokine/chemokine production and neuronal damage. Brain Res 1208:192–203. https://doi.org/10.1016/j.brainres.2008.02.093
Zaheer S, Thangavel R, Sahu SK, Zaheer A (2011a) Augmented expression of glia maturation factor in Alzheimer's disease. Neuroscience 194:227–233. https://doi.org/10.1016/j.neuroscience.2011.07.069
Zaheer S, Wu Y, Sahu SK, Zaheer A (2011b) Suppression of neuro inflammation in experimental autoimmune encephalomyelitis by glia maturation factor antibody. Brain Res 1373:230–239. https://doi.org/10.1016/j.brainres.2010.12.003
Zaheer S, Wu Y, Yang X, Thangavel R, Sahu SK, Zaheer A (2012) Efficient down-regulation of glia maturation factor expression in mouse brain and spinal cord. Neurochem Res 37(7):1578–1583. https://doi.org/10.1007/s11064-012-0753-x
Acknowledgements
Research was sponsored by the Leonard Wood Institute in cooperation with the US Army Research Laboratory and was accomplished under Cooperative Agreement Number W911NF-14-2-0034. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the Leonard Wood Institute, the Army Research Laboratory or the US Government. The US Government is authorized to reproduce and distribute reprints for Government purposes notwithstanding any copyright notation hereon. The authors express their gratitude for the Acute Effects of Neurotrauma Consortium in assisting and coordinating the conduct of this project at Fort Leonard Wood. The authors graciously acknowledge the use of the IVIS Spectrum at the Harry S. Truman VA Biomolecular Imaging Core Facility and VisualSonics Vevo 2100 Imaging System at the University of Missouri Dalton Cardiovascular Research Center. This research was also supported by NIH Grant AG048205 and VA Research Career Scientist Award to AZ.
Funding
Research was sponsored by the Leonard Wood Institute in cooperation with the US Army Research Laboratory and was accomplished under Cooperative Agreement Number W911NF-14–2-0034. This research was also supported by NIH grant AG048205 and VA Research Career Scientist Award to AZ.
Author information
Authors and Affiliations
Contributions
SPR contributed to conceptualization and experimental study design, molecular imaging data acquisition, microRNA experiments, data analysis, and original draft preparation; MEA and RT performed immunofluorescence experiments, confocal microscopy, and data analysis; GPS and DK involved in data analysis and critical reading of the manuscript; KW, OK, and KB performed experiments; BB, KK, and AK did data collection; SZ and SSI performed manuscript review and editing, project administration; TI, RG, CB, and DJ performed manuscript review and editing; AZ contributed to project supervision, project administration, funding acquisition, resources, manuscript review, editing, and final approval.
Corresponding authors
Ethics declarations
Conflict of interest
All the authors declare no conflicts of interest.
Ethical Approval
All the experimental procedures performed and described in the studies involving laboratory animals were in accordance with the ethical standards and all applicable institutional and the National Institutes of Health (NIH) guidelines for the care and use of laboratory animals were strictly followed.
Research Involving Human Rights
No human subjects were involved in the current study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raikwar, S.P., Thangavel, R., Ahmed, M.E. et al. Real-Time Noninvasive Bioluminescence, Ultrasound and Photoacoustic Imaging in NFκB-RE-Luc Transgenic Mice Reveal Glia Maturation Factor-Mediated Immediate and Sustained Spatio-Temporal Activation of NFκB Signaling Post-Traumatic Brain Injury in a Gender-Specific Manner. Cell Mol Neurobiol 41, 1687–1706 (2021). https://doi.org/10.1007/s10571-020-00937-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-020-00937-9