Preparation of Hot-Melt Extruded Dosage Form for Enhancing Drugs Absorption Based on Computational Simulation
Abstract
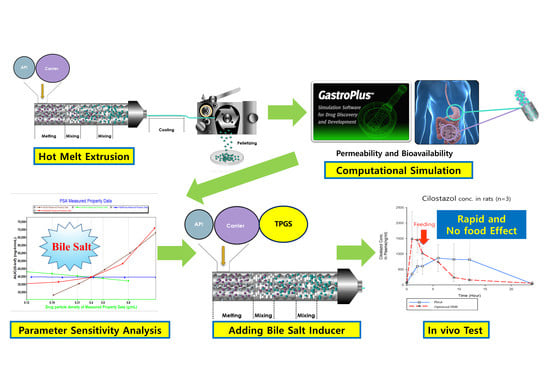
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hot-Melt Extrudates
2.3. Drug Release and Content Uniformity
2.4. Computational PBPK Simulation
2.5. Physicochemical Properties
2.6. In Vivo Test
3. Results
3.1. Processing Temperature of Hot-Melt Extrusion (HME)
3.2. The Effect of Surfactant Concentration on Dissolution Media
3.3. Optimized Hot-Melt Extruded Formulation Based on PBPK Simulation (Third Formulation)
3.4. Physicochemical Properties of the Third Formulation
3.5. In Vivo Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Amidon, G.L.; Lennernäs, H.; Shah, V.; Crison, J. A theoretical basis for a biopharmaceutical drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water-soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dai, W.G. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharm. Sin. B 2014, 4, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, I.; Renuka, V.; Lee, J.-H.; Weon, K.-Y.; Kang, C.-Y.; Lee, B.-J.; Park, J.-B. Preparation of Celecoxib Tablet by Hot Melt Extrusion Technology and Application of Process Analysis Technology to Discriminate Solubilization Effect. Pharm. Dev. Technol. 2020, 25, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Reddy Dumpa, N.; Bandari, S.; Repka, M.A. Novel Gastroretentive Floating Pulsatile Drug Delivery System Produced via Hot-Melt Extrusion and Fused Deposition Modeling 3D Printing. Pharmaceutics 2020, 12, 52. [Google Scholar] [CrossRef] [Green Version]
- Monschke, M.; Kayser, K.; Wagner, K.G. Processing of Polyvinyl Acetate Phthalate in Hot-Melt Extrusion—Preparation of Amorphous Solid Dispersions. Pharmaceutics 2020, 12, 337. [Google Scholar] [CrossRef]
- Chokshi, R.; Zia, H. Hot-melt extrusion technique: A review. Iran. J. Pharm. Res. 2004, 3, 3–16. [Google Scholar]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.B.; Battu, S.K.; McGINITY, J.W.; Martin, C. Pharmaceutical applications of hot-melt extrusion: Part I. Drug Dev. Ind. Pharm. 2007, 33, 909–926. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Boateng, J.S.; Bonnefille, M.; Aranyos, A.; Mitchell, J.; Douroumis, D. Taste masking of paracetamol by hot-melt extrusion: An in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2012, 80, 433–442. [Google Scholar] [CrossRef]
- Pawar, J.; Gokarna, V.S.; Deshpande, V.D.; Amin, P.D. Enhancement of Solubility and Stability of Itraconazole by Formation of Solid Crystal Suspensions Using Hot Melt Extrusion. Pharm. Eng. 2016, 69–71. [Google Scholar]
- Cho, H.Y.; Kang, H.A.; Park, C.H.; Kim, S.M.; Kim, D.H.; Park, S.; Kim, K.R.; Hur, H.; Lee, Y.B. Bioequivalence of Boryung torsemide tablet to Torem tablet (torasemide 10 mg) by high performance liquid chromatography/UV detector. J. Pharm. Investig. 2005, 35, 323–328. [Google Scholar]
- Rodriguez-Aller, M.; Guillarme, D.; Veuthey, J.-L.; Gurny, R. Strategies for formulating and delivering poorly water-soluble drugs. J. Drug Deliv. Sci. Technol. 2015, 30, 342–351. [Google Scholar] [CrossRef]
- Kim, T.H.; Shin, S.; Shin, B.S. Model-based drug development: Application of modeling and simulation in drug development. J. Pharm. Investig. 2018, 48, 431–441. [Google Scholar] [CrossRef]
- Ginsberg, G.; Hattis, D.; Russ, A.; Sonawane, B. Physiologically based pharmacokinetic (PBPK) modeling of caffeine and theophylline in neonates and adults: Implications for assessing children’s risks from environmental agents. J. Toxicol. Environ. Health A 2004, 67, 297–329. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.; Alberighi, O.D.C.; Läer, S.; Meibohm, B. Physiologically based pharmacokinetic (PBPK) modeling in children. Clin. Pharm. Ther. 2012, 92, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Kirtane, A.R.; Narayan, P.; Liu, G.; Panyam, J. Polymer-surfactant nanoparticles for improving oral bioavailability of doxorubicin. J. Pharm. Investig. 2017, 47, 65–73. [Google Scholar] [CrossRef]
- Liu, X.; Feng, X.; Williams, R.O.; Maurya, A. Characterization of amorphous solid dispersions. J. Pharm. Investig. 2018, 48, 19–41. [Google Scholar] [CrossRef]
- Lue, B.M.; Nielsen, F.S.; Magnussen, T.; Schou, H.M.; Kristensen, K.; Jacobsen, L.O.; Müllertz, A. Using biorelevant dissolution to obtain IVIVC of solid dosage forms containing a poorly-soluble model compound. Eur. J. Pharm. Biopharm. 2008, 69, 648–657. [Google Scholar] [CrossRef]
- Beneš, M.; Pekárek, T.; Beránek, J.; Havlíček, J.; Krejčík, L.; Šimek, M.; Tkadlecová, M.; Doležal, P. Methods for the preparation of amorphous solid dispersions—A comparative study. J. Drug Deliv. Sci. Tech. 2017, 38, 125–134. [Google Scholar] [CrossRef]
- Baek, N.; Oh, G.-H.; Park, C.; Tran, T.T.T.; Park, Y.J.; Oh, E.; Le, H.; Tran, T.T.; Park, J.-B.; Lee, B.-J. Reprecipitation of poorly water-soluble cilostazol crystals using adsorbing carriers for enhanced dissolution and physicochemical modification. J. Drug Deliv. Sci. Tech. 2018, 43, 477–486. [Google Scholar] [CrossRef]
- Lee, D.; Lim, L.A.; Jang, S.B.; Lee, Y.J.; Chung, J.Y.; Choi, J.R.; Kim, K.; Park, J.W.; Yoon, H.; Lee, J.; et al. Pharmacokinetic Comparison of Sustained- and Immediate- Release Oral Formulations of Cilostazol in Healthy Korean Subjects: A Randomized, Open-Label, 3-Part, Sequential, 2-Period, Crossover, Single-Dose, Food-Effect, and Multiple-Dose Study. Clin. Ther. 2011, 33, 2038–2053. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Ho, H.-O.; Chiou, J.-D.; Sheu, M.-T. Physical and dissolution characterization of cilostazol solid dispersions prepared by hot melt granulation (HMG) and thermal adhesion granulation (TAG) methods. Int. J. Pharm. 2014, 473, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Convention: USP 42-NF 37 The United States Pharmacopeia and National Formulary 2019; United States Pharmacopeial Convention Inc: Rockville, MD, USA, 2019.
- Liversidge, G.G.; Cundy, K.C. Particle size reduction for improvement of oral bioavailability of hydrophobic drugs: I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs. Int. J. Pharm. 1995, 125, 91–97. [Google Scholar] [CrossRef]
- Varanasi, V.S.; Veeraraghavan, S.; Potharaju, S.; Thappali, R.; Raghavan, R.; Vakkalanka, V. Validated high performance liquid chromatographic method for simultaneous determination of rosiglitazone, cilostazol, and 3, 4-dehydro-cilostazol in rat plasma and its application to pharmacokinetics. Arzneimittelforschung 2008, 58, 288–296. [Google Scholar] [CrossRef]
- Hwang, I.; Kang, C.Y.; Park, J.B. Advances in hot-melt extrusion technology toward pharmaceutical objectives. J. Pharm. Investig. 2017, 47, 123–132. [Google Scholar] [CrossRef]
- Patel, S.G.; Rajput, S.J. Enhancement of oral bioavailability of cilostazol by forming its inclusion complexes. AAPS Pharm. Sci. Tech. 2009, 10, 660–669. [Google Scholar] [CrossRef]
- Jinno, J.; Kamada, N.; Miyake, M.; Yamada, K.; Mukai, T.; Odomi, M.; Toguchi, H.; Liversidge, G.G.; Higaki, K.; Kimura, T. Effect of particle size reduction on dissolution and oral absorption of a poorly water-soluble drug, cilostazol, in beagle dogs. J. Control. Release 2006, 111, 56–64. [Google Scholar] [CrossRef]
- Patel, M.R.; Patel, M.R.; Parikh, J.R.; Patel, B.G. Formulation consideration and skin retention study of microemulsion containing tazarotene for targeted therapy of acne. J. Pharm. Investig. 2016, 46, 55–66. [Google Scholar] [CrossRef]
- Ranjita, S. Nanosuspensions: A new approach for organ and cellular targeting in infectious diseases. J. Pharm. Investig. 2013, 43, 1–26. [Google Scholar] [CrossRef]
- Saerens, L.; Dierickx, L.; Lenain, B.; Vervaet, C.; Remon, J.P.; de Beer, T. Raman spectroscopy for the in-line polymer-drug quantification and solid state characterization during a pharmaceutical hot-melt extrusion process. Eur. J. Pharm. Biopharm. 2011, 77, 158–163. [Google Scholar] [CrossRef] [Green Version]
- Park, J.B.; Kang, C.-Y.; Kang, W.-S.; Choi, H.-G.; Han, H.-K.; Lee, B.-J. New investigation of distribution imaging and content uniformity of very low dose drugs using hot-melt extrusion method. Int. J. Pharm. 2013, 458, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.T.; Nguyen, C.-H.; Nguyen, V.-D.; Nguyen, T.-H.-T.; Nguyen, V.-L.; Tran, C.-S.; Pham, T.-M.-H. Formulation and in vivo imaging evaluation of colonic targeting tablets prepared by a simple dry powder coating technique. J. Pharm. Investig. 2020, 50, 383–398. [Google Scholar] [CrossRef]
- Park, B.; Choi, H.J.; Moon, S.J.; Kim, S.J.; Bajracharya, R.; Min, J.Y.; Han, H.-K. Pharmaceutical applications of 3D printing technology: Current understanding and future perspectives. J. Pharm. Investig. 2019, 49, 575–585. [Google Scholar] [CrossRef] [Green Version]









| Parameters | First Formulation | Second Formulation | Third Formulation * |
|---|---|---|---|
| Cilostazol | 10% | 40% | 40% |
| Avicel | 45% | ||
| Kollidon | 45% | 20% | 20% |
| Soluplus | - | 40% | |
| TPGS | - | 32% | |
| Cremophor | - | 8% | |
| Processing temperature | 170 | 120 | 120 |
| Screw Speed | 60 rpm | 100 rpm | 100 rpm |
| Tissue | Volume | Tissue–Plasma Ratio | Fut/FuExt |
|---|---|---|---|
| Lung | 2.52 | 2.26 | 0.016 |
| Arterial supply | 6.72 | 0 | 0 |
| Venous return | 13.56 | 0 | 0 |
| Adipose | 12 | 7.04 | 0.001 |
| Muscle | 146.4 | 1.09 | 0.037 |
| Liver | 12.36 | 1.91 | 0.015 |
| ACAT gut | 0 | 0 | 0 |
| Spleen | 0.72 | 1.04 | 0.023 |
| Heart | 1.44 | 1.55 | 0.025 |
| Brain | 1.48 | 4.42 | 0.007 |
| Kidney | 4.44 | 1.78 | 0.014 |
| Skin | 48 | 2.56 | 0.025 |
| Reproductive organ | 3 | 1.79 | 0.014 |
| Red marrow | 2.24 | 2.36 | 0.032 |
| Yellow marrow | 4.98 | 7.04 | 0.001 |
| Rest of the body | 29.31 | 1.06 | 0.023 |
| Pletaal® | Optimized Hot-Melt Extrudates | |||||
|---|---|---|---|---|---|---|
| Cmax (ng/mL) | Tmax (h) | AUC (ng·h/mL) | Cmax (ng/mL) | Tmax (h) | AUC (ng·h/mL) | |
| 1 | 2120.22 | 12.0 | 24856.09 | 2507.32 | 1.0 | 11714.83 |
| 2 | 1357.35 | 6.0 | 10345.90 | 1158.75 | 2.0 | 8714.67 |
| 3 | 566.76 | 9.0 | 5687.10 | 1274.54 | 2.0 | 8000.12 |
| Mean ± SD | 1348.11 ± 776.77* (57.62) | 9.0 ± 3.0* (33.3) | 13629.69 ± 9997.50 * (73.35) | 1646.87 ± 747.42* (45.38) | 1.7 ± 0.6* (34.6) | 9476.54 ± 1971.07* (20.80) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-M.; Lee, S.-H.; Kang, C.-Y.; Park, J.-B. Preparation of Hot-Melt Extruded Dosage Form for Enhancing Drugs Absorption Based on Computational Simulation. Pharmaceutics 2020, 12, 757. https://doi.org/10.3390/pharmaceutics12080757
Choi S-M, Lee S-H, Kang C-Y, Park J-B. Preparation of Hot-Melt Extruded Dosage Form for Enhancing Drugs Absorption Based on Computational Simulation. Pharmaceutics. 2020; 12(8):757. https://doi.org/10.3390/pharmaceutics12080757
Chicago/Turabian StyleChoi, Sung-Min, Sung-Hoon Lee, Chin-Yang Kang, and Jun-Bom Park. 2020. "Preparation of Hot-Melt Extruded Dosage Form for Enhancing Drugs Absorption Based on Computational Simulation" Pharmaceutics 12, no. 8: 757. https://doi.org/10.3390/pharmaceutics12080757