Abstract
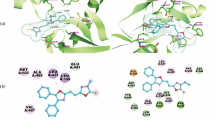
A new series of urea and thiourea bearing thiophene-2-carboxalate derivatives has been designed against protein tyrosine phosphatase 1B (PTP1B) active site, synthesized and charecterized by 1H and 13C NMR, and mass spectra. The compounds have been evaluated for in vitro anticancer activity against different cancer cell lines using the MTT colorimetric assay and doxorubicin as a standard drug. Among the tested compounds, methyl 3-methoxy-4-{4-[3-(4-methoxyphenyl)thioureido]phenyl}thiophene-2-carboxylate demonstrates the highest inhibitory activity against MCF-7, K562, HepG2, MDA-MB-231, and HeLa cell lines. The new molecular structures and their interactions with PNP1B have been evaluated by docking studies.





Similar content being viewed by others
REFERENCES
Tonks, N.K. and Muthuswamy, S.K., Cancer Cell., 2007, vol. 11, p. 214. https://doi.org/10.1016/j.ccr.2007.02.022
Bjorge, J.D., Pang, A., and Fujita, D.J., J. Biol.Chem., 2000, vol. 275, p. 41439. https://doi.org/10.1074/jbc.M004852200
Julien, S.G., Dubé, N., Read, M., Penney, J., Paquet, M., Han, Y., Kennedy, B.P., Muller, W.J., and Tremblay, M.L., Nat. Genet., 2007, vol. 39, p. 338. https://doi.org/10.1038/ng1963
Fobare, W.F., Solvibile, W.R., Robichaud, A.J., Malamas, M.S., Manas, E., Turner, J., Hu, Y., Wagner, E., Chopra, R., Cowling, R., Jin, G., and Bard, J., Bioorg. Med. Chem. Lett., 2007, vol. 17, p. 5353. https://doi.org/10.1016/j.bmcl.2007.08.010
Rajender Kumar, P., Raju, S., Satish Goud, P., Sailaja, M.,.Sarma, M.R., Om Reddy, G., Prem Kumar, M., Krishna Reddy, V.V.R.M., Suresh, T., and Hegde, P., Bioorganic Med. Chem., 2004, vol. 12, p. 1221. https://doi.org/10.1016/j.bmc.2003.11.003
Bonini, C., Chiummiento, L., De Bonis, M., Funicello, M.M., Lupattelli, P., Suanno, G., Berti, F., and Campaner, P., Tetrahedron., 2005, vol. 61, p. 6580. https://doi.org/10.1016/j.tet.2005.04.048
Battaglia, E., Bagrel, D., Migianu, E., Brault, L., Néguesque, A., and Kirsch, G., Eur. J. Med. Chem., 2005, vol. 40, p. 757. https://doi.org/10.1016/j.ejmech.2005.02.010
Buduma, K., Chinde, S., Dommati, A.K., Sharma, P., Shukla, A., Srinivas, K.V.N.S., Arigari, N.K., Khan, F., Tiwari, A.K., Grover, P., and Jonnala, K.K., Bioorg. Med. Chem. Lett., 2016, vol. 26, p. 1633. https://doi.org/10.1016/j.bmcl.2016.01.073
Romagnoli, R., Baraldi, P.G., Lopez-Cara, C., Salvador, M.K., Preti, D., Tabrizi, M.A., Balzarini, J., Nussbaumer, P., Bassetto, M., Brancale, A., Fu, X.H., Gao, Y., Li, J., Zhang, S.Z., Hamel, E., Bortolozzi, R., Basso, G., and Viola, G., Bioorg. Med. Chem., 2013, vol. 22, p. 5097. https://doi.org/10.1016/j.bmc.2013.12.030
Sztanke, M., Rzymowska, J., and Sztanke, K., Bioorg. Med. Chem., 2015, vol. 23, p. 3448. https://doi.org/
Saeed, S., Rashid, N., Jones, P.G., Ali, M., and Hussain, R., Eur. J. Med. Chem., 2010, vol. 45, p. 1323. https://doi.org/10.1016/j.ejmech.2009.12.016
Li, H.Q., Lv, P.C., Yan, T., and Zhu, H.L., Anticancer Agents. Med. Chem., 2009, vol. 9, p. 471. https://doi.org/
Lv, P.C., Li, H.Q., Sun, J., Zhou, Y., and Zhu, H.L., Bioorg. Med. Chem., 2010, vol. 18, p. 4606. https://doi.org/10.1016/j.bmc.2010.05.034
Bodige, S., Ravula, P., Gulipalli, K.C., Endoori, S., Cherukumalli, P.K.R., Seelam, N., and Chandra, J.N.N.S., Anticancer Agents Med. Chem., 2018, vol. 18, p. 891. https://doi.org/10.2174/1871520618666180209151018
Gulipalli, K.C., Bodige, S., Ravula, P., Endoori, S., Vanaja, G.R., Suresh Babu, G., Narendra Sharath Chandra, J.N., and Seelam, N., Bioorg. Med. Chem. Lett., 2017, vol. 27, p. 3558. https://doi.org/
Ravula, P., Bodige, S., Gulipalli, K.C., Vamaraju, H.B., Narendra Sharath Chandra, J.N., and Paturi, M., J. Heterocycl. Chem., 2018, vol. 55, p. 1313. https://doi.org/
Ravula, P. Vamaraju, H.B., Paturi, M., and Sharath Chandra, J.N.N., Arch. Pharm. (Weinheim)., 2018, vol. 351, p. 1. https://doi.org/
Parameshwar, R., Harinadha Babu, V., Manichandrika, P., Narendra Sharath Chandra, J.N., and Swetha, K., EXCLI J., 2016, vol.15, p. 187. https://doi.org/
ACKNOWLEDGMENTS
The authors would like to thank the management of AMRI Hyderabad research centre for giving an opportunity to carry out this research. The authors are also thankful to Sai Innotech Labs Pvt.Ltd.for providing synthesis facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Gulipalli, K.C., Ravula, P., Bodige, S. et al. Synthesis and Anticancer Activity of Novel Urea and Thiourea Bearing Thiophene-2-carboxalate Derivatives. Russ J Gen Chem 90, 1336–1344 (2020). https://doi.org/10.1134/S1070363220070221
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363220070221