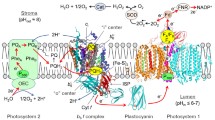
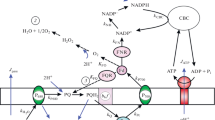
Abstract
The significance of temperature-dependent regulation of photosynthetic apparatus (PSA) is determined by the fact that plant temperature changes with environmental temperature. In this work, we present a brief overview of temperature-dependent regulation of photosynthetic processes in class B chloroplasts (thylakoids) and analyze these processes using a computer model that takes into account the key stages of electron and proton transport coupled to ATP synthesis. The rate constants of partial reactions were parametrized on the basis of experimental temperature dependences of partial photosynthetic processes: (1) photosystem II (PSII) turnover and plastoquinone (PQ) reduction, (2) the plastoquinol (PQH2) oxidation by the cytochrome (Cyt) b6f complex, (3) the ATP synthase activity, and (4) the proton leak from the thylakoid lumen. We consider that PQH2 oxidation is the rate-limiting step in the intersystem electron transport. The parametrization of the rate constants of these processes is based on earlier experimental data demonstrating strong correlations between the functional and structural properties of thylakoid membranes that were probed with the lipid-soluble spin labels embedded into the membranes. Within the framework of our model, we could adequately describe a number of experimental temperature dependences of photosynthetic reactions in thylakoids. Computer modeling of electron and proton transport coupled to ATP synthesis supports the notion that PQH2 oxidation by the Cyt b6f complex and proton pumping into the lumen are the basic temperature-dependent processes that determine the overall electron flux from PSII to molecular oxygen and the net ATP synthesis upon variations of temperature. The model describes two branches of the temperature dependence of the post-illumination reduction of \( {\text{P}}_{700}^{ + } \) characterized by different activation energies (about 60 and ≤ 3.5 kJ mol−1). The model predicts the bell-like temperature dependence of ATP formation, which arises from the balance of several factors: (1) the thermo-induced acceleration of electron transport through the Cyt b6f complex, (2) deactivation of PSII photochemistry at sufficiently high temperatures, and (3) acceleration of the passive proton outflow from the thylakoid lumen bypassing the ATP synthase complex. The model describes the temperature dependence of experimentally measured parameter P/2e, determined as the ratio between the rates of ATP synthesis and pseudocyclic electron transport (H2O → PSII → PSI → O2).











Similar content being viewed by others
Abbreviations
- CBC:
-
Calvin–Benson cycle
- DGDG:
-
Digalactosyldiacylglycerol
- EPR:
-
Electron paramagnetic resonance
- ETC:
-
Electron transport chain
- Fd:
-
Ferredoxin
- FNR:
-
Ferredoxin-NADP-oxidoreductase
- ISP:
-
Iron–sulfur protein
- MGDG:
-
Monogalactosyldiacylglycerol
- ODE:
-
Ordinary differential equations
- Pc:
-
Plastocyanin
- PG:
-
Phosphatidylglycerol
- pmf :
-
Proton-motive force
- PSI and PSII:
-
Photosystem I and photosystem II, respectively
- PQ and PQH2 :
-
Plastoquinone and plastoquinol (fully reduced form of PQ), respectively
- P700 :
-
Special chlorophyll pair in PSI, primary electron donor in PSI
- P680 :
-
Special chlorophyll pair in PSII, primary electron donor in PSII
- QA and QB :
-
Primary and secondary plastoquinone molecules bound to PSII
- SQDG:
-
Sulfoquinovosyldiacylglycerol
- T :
-
Temperature in Kelvin scale
- t :
-
Temperature in Celsius scale
- τ :
-
Time
- WOC:
-
Water-oxidizing complex
- 5-SASL:
-
Spin probe 5-doxylstearate
References
Agalarov R, Brettel K (2003) Temperature dependence of biphasic forward electron transfer from the phylloquinone(s) A1 in photosystem I: only the slower phase is activated. Biochim Biophys Acta 1604:7–12
Albertsson PE (2001) A quantitative model of the domain structure of the photosynthetic membrane. Trends Plant Sci 6:349–354
Allakhverdiev SI, Kreslavski VD, Klimov VV, Los DA, Carpentier R, Mohanty P (2008) Heat stress: an overview of molecular responses in photosynthesis. Photosynth Res 98:541–550
Allen DJ, Ort DR (2001) Impact of chilling temperatures on photosynthesis in warm climate plants. Trends Plant Sci 6:36–42
Aloia RA, Boggs JM (eds) (1985) Membrane fluidity in biology. Academic Press, New York, pp 147–208
Anderson JM (1982) Distribution of the cytochromes of spinach chloroplasts between the appressed membranes of grana stacks and stroma-exposed thylakoid regions. FEBS Lett 138:62–66
Ariga T, Muneyuki E, Yoshida M (2007) F1-ATPase rotates by an asymmetric, sequential mechanism using all three catalytic subunits. Nat Struct Mol Biol 14:841–846
Arnold A, Nikoloski Z (2011) A quantitative comparison of Calvin-Benson cycle models. Trends Plant Sci 16:676–683
Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Ann Rev Plant Physiol Plant Mol Biol 50:601–639
Ataullakhanov FI, Pichugin AV (1981) Modification of luciferin-luciferase method for ATP assay in erythrocytes. Biophysics (USSR) 26:81–86
Badger MR, Von Caemmerer S, Ruuska S, Nakano H (2000) Electron flow to oxygen in higher plants and algae: rates and control of direct photoreduction (Mehler reaction) and rubisco oxygenase. Philos Trans R Soc London 355:1433–1446
Bakker-Grunwald T, van Dam K (1974) On the mechanism of activation of the ATPase in chloroplasts. Biochim Biophys Acta 347:290–298
Bald D, Noji H, Yoshida M, Hirono-Hara Y, Hisabori T (2001) Redox regulation of the rotation of F1-ATP synthase. J Biol Chem 276:39505–39507
Baniulis D, Yamashita E, Zhang H, Hasan SS, Cramer WA (2008) Structure–function of the cytochrome b6f complex. Photochem Photobiol 84:1349–1358
Barber J, Ford RC, Mitchell RA, Millner PA (1984) Chloroplast thylakoid membrane fluidity and its sensitivity to temperature. Planta 161:375–380
Bendall DS, Manasse RS (1995) Cyclic photophosphorylation and electron transport. Biochim Biophys Acta 1229:23–38
Benkov MA, Yatsenko AM, Tikhonov AN (2019) Light acclimation of shade-tolerant and sun-resistant Tradescantia species: photochemical activity of PSII and its sensitivity to heat treatment. Photosynth Res 139:203–214
Benson BB, Krause Jr D (1984) The concentration and isotopic fractionation of oxygen dissolved in freshwater and seawater in equilibrium with the atmosphere. Limnol Oceanogr 29:620–632
Berliner LJ (ed) (1976) Spin labeling: theory and applications. Academic Press, New York-London
Berry J, Björkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31:491–543
Boardman NK (1977) Comparative photosynthesis of sun and shade plants. Ann Rev Plant Physiol 28:335–377
Boudiere L, Michaud M, Petroutsos D, Rebeille F, Falconet D, Bastien O, Roy S, Finazzi G, Rolland N, Jouhet J et al (2014) Glycerolipids in photosynthesis: composition, synthesis and trafficking. Biochim Biophys Acta 1837:470–480
Boyer PD (1997) The ATP synthase—a splendid molecular machine. Annu Rev Biochem 66:717–749
Brandt U (1996) Bifurcated ubihydroquinone oxidation in the cytochrome bc1 complex by proton-gated charge transfer. FEBS Lett 387:1–6
Brettel K (1997) Electron transfer and arrangement of the redox cofactors in photosystem I. Biochim Biophys Acta 1318:322–373
Brettel K, Leibl W (2001) Electron transfer in photosystem I. Biochim Biophys Acta 1507:100–114
Cardona T, Sedoud A, Cox N, Rutherford AW (2012) Charge separation in photosystem II: a comparative and evolutionary overview. Biochim Biophys Acta 1817:26–43
Chance B, Williams GR (1956) The respiratory chain and oxidative phosphorylation. Adv Enzymol 17:65–134
Cherepanov DA, Milanovsky GE, Petrova AA, Tikhonov AN, Semenov AYu (2017) Electron transfer through the acceptor side of Photosystem I: interaction with exogenous acceptors and molecular oxygen. Biochemistry (Moscow) 82:1249–1268
Clever HL, Battino R, Miyamoto H, Yampolski Y, Young CL (2014) IUPAC-NIST Solubility Data Series. 103. Oxygen and Ozone in Water, Aqueous Solutions, and Organic Liquids (Supplement to Solubility Data Series Volume 7). J Phys Chem Ref Data 43(3)
Cramer WA, Hasan SS (2016) Structure-function of the cytochrome b6f lipoprotein complex. In cytochrome complexes: evolution, structures, energy transduction, and signaling. Adv Photosynth Respiration 41:177–207
Cramer WA, Zhang H, Yan J, Kurisu G, Smith JL (2006) Transmembrane traffic in the Cytochrome b6f complex. Ann Rev Biochem 75:769–790
Cramer WA, Hasan SS, Yamashita E (2011) The Q cycle of cytochrome bc complexes: a structure perspective. Biochim Biophys Acta 1807:788–802
Crofts AR (2004) Proton-coupled electron transfer at the Qo-site of the bc1 complex controls the rate of ubihydroquinone oxidation. Biochim Biophys Acta 1655:77–92
Crofts AR, Wang Z (1989) How rapid are the internal reactions of the ubiquinol:cytochrome c2 oxidoreductase? Photosynth Res 22:69–87
Crofts AR, Guergova-Kuras M, Kuras R, Ugulava N, Li J, Hong S (2000) Proton-co;upled electron transfer at the Qo-site: what type of mechanism can account for the high activation barrier? Biochim Biophys Acta 1459:456–466
Crofts AR, Hong S, Wilson C, Burton R, Victoria D, Harrison C, Schulten K (2013) The mechanism of ubihydroquinone oxidation at the Qo-site of the cytochrome bc1 complex. Biochim Biophys Acta 1827:1362–1377
Dau H, Haumann M (2008) The manganese complex of photosystem II in its reaction cycle—basic framework and possible realization at the atomic level. Coord Chem Rev 252:273–295
Davis GA, Rutherford AW, Kramer DM (2017) Hacking the thylakoid proton motive force for improved photosynthesis: modulating ion flux rates that control proton motive force partitioning into Δψ and ΔpH. Philos Trans R Soc B 372:20160381
de Wijn R, van Gorkom HJ (2001) Kinetics of electron transfer from QA to QB in photosystem II. Biochemistry 40:11912–11922
Deamer DW (1987) Proton permeation of lipid bilayers. J Bioenerg Biomembr 19:457–479
Dekker JP, Boekema EJ (2005) Supermolecular organization of the thylakoid membrane proteins in green plants. Biochim Biophys Acta 1706:12–39
Demmig-Adams B, Cohu CM, Muller O et al (2012) Modulation of photosynthetic energy conversion efficiency in nature: from seconds to seasons. Photosynth Res 113:75–88
Díaz-Quintana A, Leibl W, Bottin H, Sétif P (1998) Electron transfer in photosystem I reaction centers follows a linear pathway in which iron–sulfur cluster FB is the immediate electron donor to soluble ferredoxin. BioChemistry 37:3429–3439
Diez M, Zimmermann B, Borsch M, Konig M, Schweinberger E, Steigmiller S, ReuterR Felekyan S, Kudryavtsev V, Seidel CA, Graber P (2004) Proton-powered subunit rotation in single membrane-bound F0F1-ATP synthase. Nat Struct Mol Biol 11:135–141
Dubinskii AYu, Tikhonov AN (1995) Mathematical simulation of the light-induced uptake of protons by chloroplasts upon various mechanisms of proton leak through the thylakoid membrane. Biophysics 40:365–371
Dubinskii AYu, Tikhonov AN (1997) Mathematical model of thylakoid as the distributed heterogeneous system of electron and proton transport. Biophysics 42:644–660
Edwards G, Walker D (1983) C3, C4: mechanisms, and cellular and environmental regulation, of photosynthesis. Univ of California Press, Berkeley
Fillingame RH, Jiang W, Dmitriev OY (2000) Coupling H+ transport to rotary catalysis in F-type ATP synthases: structure and organization of the transmembrane rotary motor. J Exp Biol 203:9–17
Ford RC, Barber J (1983) Incorporation of sterol into chloroplast thylakoid membranes and its effect on fluidity and function. Planta 158:35–41
Ford RC, Chapman DJ, Barber J, Pedersen JZ, Cox RP (1982) Fluorescence polarization and spin-label studies of the fluidity of stromal and granal chloroplast membranes. Biochim Biophys Acta 681:145–151
Foyer CH, Neukermans J, Queval G, Noctor G, Harbinson J (2012) Photosynthetic control of electron transport and the regulation of gene expression. J Exp Bot 63:637–1661
Gong X-S, Chung S, Fernandez-Velasco JG (2001) Electron transfer and stability of the cytochrome b6f complex in a small domain deletion mutant of cytochrome f. J Biol Chem 276:24365–24371
Gounaris K, Brain APR, Quinn PJ, Williams WP (1984) Structural reorganization of chloroplast thylakoid membranes in response to heat-stress. Biochim Biophys Acta 766:198–208
Griffith OH, Jost PC (1976) Lipid spin labels in biological membranes. Spin labeling: theory and applications. Academic Press, New York, pp 456–524
Gutknecht J (1987) Weak electrolyte transport across biological membranes. General principles. J Bioenerg Biomembr 19:427–455
Haehnel W (1973) Electron transport between plastoquinone and chlorophyll aI. Biochim Biophys Acta 305:618–631
Haehnel W (1976) The reduction kinetics of chlorophyll a1 as an indicator of for proton uptake between the light reactions in chloroplasts. Biochim Biophys Acta 440:506–521
Haehnel W (1984) Photosynthetic electron transport in higher plants. Annu Rev Plant Physiol 35:659–693
Hasan SS, Cramer WA (2012) On rate limitation of electron transfer in the photosynthetic b6f complex. Phys Chem Chem Phys 14:13856–13860
Hasan SS, Cramer WA (2014) Internal lipid architecture of the hetero-oligomeric cytochrome b6f complex. Structure 22:1–8
Hasan SS, Yamashita E, Baniulis D, Cramer WA (2013a) Quinone-dependent proton transfer pathways in the photosynthetic cytochrome b6f complex. Proc Natl Acad Sci USA 110:4293–4302
Hasan SS, Stofleth JT, Yamashita E, Cramer WA (2013b) Lipid-induced conformational changes within the cytochrome b6f complex of oxygenic photosynthesis. Biochemistry 52:2649–2654
Heise K-P, Harnischfeger G (1978) Correlation between photosynthesis and plant lipid composition. Z Naturforsch 33:537–546
Hirano M, Satoh K, Katoh S (1981) The effect on photosynthetic electron transport of temperature-dependent changes in the fluidity of the thylakoid membrane in a thermophilic blue-green alga. Biochim Biophys Acta 635:476–487
Hong SJ, Ugulava N, Guergova-Kuras M, Crofts AR (1999) The energy landscape for ubihydroquinone oxidation at the Qo-site of the bc1 complex in Rhodobacter sphaeroides. J Biol Chem 274:33931–33944
Hope AB (2000) Electron transfers amongst cytochrome f, plastocyanin and photosystem I: kinetics and mechanisms. Biochim Biophys Acta 1456:5–26
Horton P (2012) Optimization of light harvesting and photoprotection: molecular mechanisms and physiological consequences. Phil Trans R Soc B 367:3455–3465
Hu S, Ding Y, Zhu C (2020) Sensitivity and responses of chloroplasts to heat stress in plants. Front Plant Sci 11:375. https://doi.org/10.3389/fpls.2020.00375
Igamberdiev AU (2011) Computational models of photosynthesis. BioSystems 103:113–314
Inout H (1978) Break points in Arrhenius plots of the Hill reaction of spinach chloroplast fragments in the temperature range from -25 to 25°C. Plant Cell Physiol 19:355–363
Ivanov BN (1993) Stoichiometry of proton uptake by thylakoids during electron transport in chloroplasts. In: Abrol YP, Mohanty P, Govindjee G (eds) Photosynthesis: Photoreactions to Plant Productivity. Springer, Dordrecht, pp 108–128
Johnson MP, Ruban AV (2014) Rethinking the existence of a steady-state Δψ component of the proton motive force across plant thylakoid membranes. Photosynth Res 119:233–242
Junge W, Nelson N (2015) ATP synthase. Annu Rev Biochem 83:631–657
Junge W, Lill H, Engelbrecht S (1997) ATP synthase: an electrochemical transducer with rotatory mechanics. Trends Biochem Sci 22:420–423
Karavaev VA, Kukushkin AK (1993) Theoretical model of the light and dark stages of photosynthesis: the regulation problem. Biophysics 38:958–975
Kern J, Zouni A, Guskov A, Krauβ N (2009) Lipid in the structure of photosystem I, photosystem II and the cytochrome b6f complex. In: Wada H, Murata N (eds) Lipids in Photosynthesis: Essential and Regulatory Functions. Springer, Dordrecht, pp 203–242
Kirchhoff H (2008) Significance of protein crowding, order and mobility for photosynthetic membrane functions. Biochem Soc Trans 36:967–970
Kirchhoff H (2014) Diffusion of molecules and macromolecules in thylakoid membranes. Biochim Biophys Acta 1837:495–502
Kirchhoff H, Horstmann S, Weis E (2000) Control of the photosynthetic electron transport by PQ diffusion microdomains in thylakoids of higher plants. Biochim Biophys Acta 1459:148–168
Kirchhoff H, Hall C, Wood M, Herbstová M, Tsabari O, Nevo R, Charuvi D, Shimoni E, Reich Z (2011) Dynamic control of protein diffusion within the granal thylakoid lumen. Proc Natl Acad Sci USA 108:20248–20253
Kraayenhof R, Katan MB, Grunwald T (1971) The effect of temperature on energy-linked functions in chloroplasts. FEBS Lett 19:5–10
Kramer DM, Sacksteder CA, Cruz JA (1999) How acidic is the lumen? Photosynth Res 60:151–163
Kukushkin AK, Tikhonov AN (1988) Lectures on Biophysics of Photosynthesis in Higher Plants. Moscow University Press, Moscow (in Russian)
Kumamoto J, Raison JK, Lyons JM (1971) Temperature “breaks” in Arrhenius plots: a thermodynamic consequence of a phase change. J Theor Biol 31:47–51
Kuvykin IV, Vershubskii AV, Ptushenko VV, Tikhonov AN (2008) Oxygen as an alternative electron acceptor in the photosynthetic electron transport chain of C3 plants. Biochemistry (Moscow) 73:1063–1075
Laisk A, Nedbal N, Govindjee (eds) (2009) Photosynthesis in silico. Understanding complexity from molecules to ecosystems. Springer, Dordrecht
Lazár D, Schansker G (2009) Models of chlorophyll a fluorescence transients. In: Laisk A, Nedbal L, Govindjee S (eds) Photosynthesis in silico: understanding complexity from molecules to ecosystems. Advances in photosynthesis and respiration, vol 29. Springer, Dordrecht, pp 85–123
Lee AJ (1977) Lipid phase transitions and phase diagrams. I. Lipid phase transitions. Biochim Biophys Acta 472:237–281
Li Z, Wakao S, Fischer BB, Niyogi KK (2009) Sensing and responding to excess light. Annu Rev Plant Biol 60:239–260
Ligeza A, Tikhonov AN, Hyde JS, Subczynski WK (1998) Oxygen permeability of thylakoid membranes: electron paramagnetic resonance spin labeling study. Biochim Biophys Acta 1365:453–463
Link TA (1997) The role of the “Rieske” iron sulfur protein in the hydroquinone oxidation (Qp) site of the cytochrome bc1 complex: the “proton-gated affinity change” mechanism. FEBS Lett 412:257–264
Los DA, Murata N (2004) Membrane fluidity and its role in the perception of environmental signals. Biochim Biophys Acta 1666:142–157
Los DA, Mironov KS, Allakhverdiev SI (2013) Regulatory role of membrane fluidity in gene expression and physiological functions. Photosynth Res 116:489–509
Lubitz W, Chrysina M, Cox N (2019) Water oxidation in photosystem II. Photosynth Res 142:105–125
Lutova MI, Tikhonov AN (1983) Aftereffect of high temperature on photosynthesis and electron transport in wheat leaves. Biophysics 28:284–287
Lutova MI, Tikhonov AN (1988) Comparative study of temperature effects on the mobility of lipid- soluble spin label in thylakoid membranes of melon and cucumber chloroplasts. Biophysics 33:460–464
Luzikov VN, Novikova LA, Tikhonov AN, Zubatov AS (1983) Correlation between the rate of proteolysis of mitochondrial translation products and fluidity of the mitochondrial inner membrane of Saccharomices cervisiae. Biochem J 214:785–794
Luzikov VN, Novikova LA, Zubatov AS, Tikhonov AN (1984) Physical state of the mitochondrial inner membrane as a factor controlling the proteolysis of mitochondrial translational products in yeasts. Biochim Biophys Acta 775:22–30
Maksimov EG, Mironov KS, Trofimova MS, Nechaeva NL, Todorenko DA, Klementiev KE, Tsoraev GV, Tyutyaev EV, Zorina AA, Feduraev PV, Allakhverdiev SI, Paschenko VZ, Los DA (2017) Membrane fluidity controls redox-regulated cold stress responses in cyanobacteria. Photosynth Res 133:215–223
Malenkova IV, Kuprin SP, Davidov RM, Blumenfeld LA (1982) pH-jump-induced ADP phosphorylation in mitochondria. Biochim Biophys Acts 682:179–183
Mamedov M, Govindjee Nadtochenko V, Semenov A (2015) Primary electron transfer processes in photosynthetic reaction centers from oxygenic organisms. Photosynth Res 125:51–63
Margolis LB, Tikhonov AN, Vasilieva EYu (1980) Platelet adhesion to fluid and solid phospholipid membranes. Cell 19:189–194
McConnell HM (1976) Molecular motion in biological membranes. Spin labeling: theory and applications. Academic Press, New York, pp 525–561
Melnichenko NA, Koltunov AM, Vyskrebentsev AS, Bazhanov AV (2008) The temperature dependence of the solubility of oxygen in sea water according to the pulsed NMR data. Russ J Phys Chem 82:746–752
Meyer B, Schlodder E, Dekker JP, Witt HT (1989) O2 evolution and Chl a +II (P-680+) nanosecond reduction kinetics in single flashes as a function of pH. Biochim Biophys Acta 974:36–43
Milanovsky GE, Petrova AA, Cherepanov DA, Semenov AY (2017) Kinetic modeling of electron transfer reactions in photosystem I complexes of various structures with substituted quinone acceptors. Photosynth Res 133:185–199
Mitchell P (1976) Possible molecular mechanisms of the protonmotive function of cytochrome systems. J Theor Biol 62:327–367
Mizusawa N, Wada H (2012) The role of lipids in photosystem II. Biochim Biophys Acta 1817:194–208
Möbius K, Savitsky A (2009) High-field EPR spectroscopy on proteins and their model systems: characterization of transient paramagnetic states. RSC Publishing, London
Moon BY, Higashi S, Gombos Z, Murata N (1995) Unsaturation of the membrane lipids of chloroplasts stabilizes the photosynthetic machinery against low-temperature photoinhibition in transgenic tobacco plants. Proc Natl Acad Sci USA 92:6219–6223
Morales A, Yin X, Harbinson J, Driever SM, Molenaar J, Kramer DM, Struik P (2018) In silico analysis of the regulation of the photosynthetic electron transport chain in C3 plants. Plant Physiol 176:1247–1261
Moser CC, Keske JM, Warncke K, Farid RS, Dutton PL (1992) Nature of biological electron transfer. Nature 355:796–802
Murata N, Fork DC (1977) Temperature dependence of the light-induced spectral shift of carotenoids in Cvanidium caldarium and higher plant leaves. Evidence for an effect of the physical phase of chloroplast membrane lipids on the permeability of the membranes to ions. Biochim Biophys Acta 461:365–378
Nagle JF (1987) Theory of passive proton conductance in lipid bilayers. J Bioenerg Biomembr 19:413–426
Nelson N, Yocum CF (2006) Structure and function of photosystems I and II. Annu Rev Plant Biol 57:521–565
Nie GY, Baker NR (1991) Modifications to thylakoid composition during development of maize leaves at low growth temperatures. Plant Physiol 95:184–191
Nievola CC, Carvalho CP, Carvalho V, Rodrigues E (2017) Rapid responses of plants to temperature changes. Temperature 4:371–405
Nishimura M, Ito T, Chance B (1962) Studies on bacterial photophosphorylation III. A sensitive and rapid method of determination of photophosphorylation. Biochim Biophys Acta 59:177–182
Niu Y, Xiang Y (2018) An overview of biomembrane functions in plant responses to high-temperature stress. Front Plant Sci 9:915
Nolan WG (1980) Effect of temperature on electron transport activities of isolated chloroplasts. Plant Physiol 66:234–237
Nolan WG (1981) Effect of temperature on proton efflux from isolated chloroplast thylakoids. Plant Physiol 67:1259–1263
Nolan WG, Smillie RM (1976) Multi-temperature effects on Hill reaction activity of barley chloroplasts. Biochim Biophys Acta 440:461–475
Nolan WG, Smillie RM (1977) Temperature-induced changes in Hill activity of chloroplasts isolated from chilling-sensitive and chilling-resistant plants. Plant Physiol 59:1141–1145
Ogawa S, Shen C, Castillo CLA (1980) NMR study of the cross-membrane pH gradient induced by ATP hydrolysis in mitochondria. Biochim Biophys Acta 590:159–169
Ort DR, Baker NR (2002) A photoprotective role for O2 as an alternative electron sink in photosynthesis? Curr Opin Plant Biol 5:193–198
Osyczka A, Moser CC, Dutton L (2005) Fixing the Q cycle. Trends Biochem Sci 30:176–182
Page CC, Moser CC, Chen X et al (1999) Natural engineering principles of electron tunnelling in biological oxidation-reduction. Nature 402:47–52
Quinn PJ, Williams WP (1978) Plant lipids and their role in membrane function. Progr Biophys Molec Biol 34:107–173
Razeghifard MR, Klughammer C, Pace RJ (1997) Electron paramagnetic resonance kinetic studies of the S states in spinach thylakoids. Biochemistry 36:86–92
Reeves SG, Hall DO, West J (1972) Correlation of the stoichiometry of photophosphorylation with the integrity of isolated spinach chloroplasts. In: Forti G, Avron M, Melandri A (eds) Photosynthesis, two centuries after its discovery by Joseph Priestley. Springer, Dordrecht
Rigoulet M, Leverve X, Fontaine E, Ouhabi R, Guérin B (1998) Quantitative analysis of some mechanisms affecting the yield of oxidative phosphorylation: dependence upon both fluxes and forces. Mol Cell Biochem 184:35–52
Riznichenko GY, Belyaeva NE, Kovalenko IB, Rubin AB (2009) Mathematical and computer modeling of primary photosynthetic processes. Biophys 54:10–22
Romanovsky YuM, Tikhonov AN (2010) Molecular energy transducers of the living cell. Proton ATP synthase: a rotating molecular motor. Phys Usp 53:893–914
Rubin A, Riznichenko G (2014) Mathematical biophysics. Series: biological and medical physics, biomedical engineering, XV
Sanderson DG, Anderson LB, Gross EL (1986) Determination of the redox potential and diffusion coefficient of the protein plastocyanin using optically transparent filar electrodes. Biochim Biophys Acts 852:269–278
Santabarbara S, Redding KE, Rappaport F (2009) Temperature dependence of the reduction of P +700 by tightly bound plastocyanin in vivo. Biochemistry 48:10457–10466
Sarcina M, Murata N, Tobin MJ, Mullineaux CW (2003) Lipid diffusion in the thylakoid membranes of the cyanobacterium Synechococcus sp.: effect of fatty acid desaturation. FEBS Lett 553:295–298
Sawada S, Miyachi S (1974) Effects of growth temperature on photosynthetic carbon metabolism in green plants I. Photosynthetic activities of various plants acclimatized to varied temperatures. Plant Cell Physiol 15:111–120
Schneider AR, Geissler PL (2013) Coexistance of fluid and crystalline phases of proteins in photosynthetic membranes. Biophys J 105:1161–1170
Schuurmans JJ, Kraayenhof R (1983) Energy-regulated functional transitions of chloroplast ATPase. Photochem Photobiol 37:85–91
Seelert H, Poetsch A, Dencher NA, Engel A, Stahlberg H, Müller DJ (2000) Structural biology. Proton-powered turbine of a plant motor. Nature 405:418–419
Setif PQY, Bottin H (1994) Laser flash absorption spectroscopy study of ferredoxin reduction by Photosystem I in Synechocystis sp. PCC 6803: Evidence for submicrosecond and microsecond kinetics. Biochemistry 33:8495–8504
Shelaev IV, Gostev FE, Mamedov MD, Sarkisov OM, Nadtochenko VA, Shuvalov VA, Semenov AYu (2010) Femtosecond primary charge separation in photosystem I. Biochim Biophys Acta 1797:1410–1420
Shneyour A, Raison JK, Smillie RM (1973) The effect of temperature on the rate of photosynthetic electron transfer in chloroplasts of chilling-sensitive and chilling-resistant plants. Biochim Biophys Acta 292:152–161
Sigfridsson K (1998) Plastocyanin, an electron transfer protein. Photosynth Res 57:1–28
Siggel U (1976) The function of plastoquinone as electron and proton carrier in photosynthesis. Bioelectrochem Bioenerg 3:302–318
Staehelin LA (2003) Chloroplast structure: from chlorophyll granules to supra-molecular architecture of thylakoid membranes. Photosynth Res 76:185–196
Stiehl HH, Witt HT (1969) Quantitative treatment of the function of plastoquinone in photosynthesis. Z Naturforsch B 24:1588–1598
Stirbet A, Govindjee G (2016) The slow phase of chlorophyll a fluorescence induction in silico: origin of the S-M fluorescence rise. Photosynth Res 130:193–213
Stirbet A, Riznichenko GY, Rubin AB, Govindjee G (2014) Modeling chlorophyll a fluorescence transient: relation to photosynthesis. Biochemistry (Moscow) 79:291–323
Stirbet A, Lazár D, Guo Y, Govindjee G (2019) Photosynthesis: basics, history, and modeling. Ann Bot. https://doi.org/10.1093/aob/mcz171
Strand DD, Fisher N, Kramer DM (2016) Distinct energetics and regulatory functions of the two major cyclic electron flow pathways in chloroplasts. In: Kirchhoff H (ed) Chloroplasts: current research and future trends. Caister Academic Press, Norfolk, pp 89–100
Suslichenko IS, Tikhonov AN (2019) Photo-reducible plastoquinone pools in chloroplasts of Tradescentia plants acclimated to high and low light. FEBS Lett 593:788–798
Tietz S, Puthiyaveetil S, Enlow HM, Yarbrough R, Wood M, Semchonok DA, Lowry T, Li Z, Jahns P, Boekema EJ, Lenhert S, Niyogi KK, Kirchhoff H (2015) Functional implications of Photosystem II crystal formation in photosynthetic membranes. J Biol Chem 290:14091–14106
Tikhonov AN (2012) Energetic and regulatory role of proton potential in chloroplasts. Biochemistry (Moscow) 77:956–974
Tikhonov AN (2013) pH-Dependent regulation of electron transport and ATP synthesis in chloroplasts. Photosynth Res 116:511–534
Tikhonov AN (2014) The cytochrome b6f complex at the crossroad of photosynthetic electron transport pathways. Plant Physiol Biochem 81:163–183
Tikhonov AN (2015) Induction events and short-term regulation of electron transport in chloroplasts: an overview. Photosynth Res 125:65–94
Tikhonov AN (2016) Modeling electron and proton transport in chloroplasts. In: Kirchhoff H (ed) Chloroplasts: current research and future trends. Caister Academic Press, Norfolk, pp 101–134
Tikhonov AN (2017) Photosynthetic electron and proton transport in chloroplasts: EPR study of ΔpH generation, an overview. Cell Biochem Biophys 75:421–432
Tikhonov AN (2018) The cytochrome b6f complex: biophysical aspects of its functioning in chloroplasts. In: Harris JR, Boekema EJ (eds) Membrane protein complexes: structure and function, subcellular biochemistry, vol 87. Springer, Singapore, pp 287–328
Tikhonov AN (2020) Structure-function relationships in chloroplasts: EPR study of temperature-dependent regulation of photosynthesis, an overview. In: JR Shen, K Satoh, SI Allakhverdiev (eds) Photosynthesis: molecular approaches to solar energy conversion.
Tikhonov AN, Blumenfeld LA (1985) Hydrogen ions concentration in subcellular systems: physical meaning and the methods for determination. 30:527–537
Tikhonov AN, Subczynski WK (2005) Application of spin labels to membrane bioenergetics (photosynthetic systems of higher plants), chapter 8. In: Eaton SS, Eaton GR, Berliner LJ (eds) Biological magnetic resonance, vol 23. Biomedical EPR—part A: free radicals, metals, medicine, and physiology. Kluwer Academic Publishers, New York, pp 147–194
Tikhonov AN, Vershubskii AV (2014) Computer modeling of electron and proton transport in chloroplasts. BioSystems 121:1–21
Tikhonov AN, Vershubskii AV (2017) Connectivity between electron transport complexes and modulation of photosystem II activity in chloroplasts. Photosynth Res 133:103–114
Tikhonov AN, Khomutov GB, Ruuge EK (1980) Electron spin resonance study of electron transport in photosynthetic systems. IX. Temperature dependence of the kinetics of P700 redox transients in bean chloroplasts induced by flashes with different duration. Mol Biol (Moscow) 14:157–172
Tikhonov AN, Khomutov GB, Ruuge EK, Blumenfeld LA (1981) Electron transport control in chloroplasts. Effects of photosynthetic control monitored by the intrathylakoid pH. Biochem Biophys Acta 637:321–333
Tikhonov AN, Timoshin AA, Blumenfeld LA (1983) Electron transport kinetics, proton transfer, photophosphorylation in chloroplasts, and their relation to thermo-induced structural changes in the thylakoid membrane. Mol Biol (Moscow) 17:1236–1248
Tikhonov AN, Khomutov GB, Ruuge EK (1984) Electron transport control in chloroplasts. Effects of magnesium ions on the electron flow between two photosystems. Photobiochem Photobiophys 8:261–269
Tikhonov AN, Agafonov RV, Grigor’ev IA, Kirilyuk IA, Ptushenko VV, Trubitsin BV (2008) Spin-probes designed for measuring the intrathylakoid pH in chloroplasts. Biochim BiophysActa 1777:285–294
Timoshin AA, Tikhonov AN, Blumenfeld LA (1984) Thermoinduced structural changes in ATP-synthase as a factor of energy transduction control in chloroplasts. Biophysics 29:338–340
Torres-Pereira J, Mehlhorn R, Keith AD, Packer L (1974) Changes in membrane lipid structure of illuminated chloroplasts—studies with spin labeled and freeze-fractured membranes. Arch Biochem Biophys 160:90–99
Tremmel IG, Kirchhoff H, Weis E, Farquhar GD (2003) Dependence of plastoquinol diffusion on the shape, size, and density of integral thylakoid proteins. Biochim Biophys Acta 1603:97–109
Trubitsin BV, Tikhonov AN (2003) Determination of a transmembrane pH difference in chloroplasts with a spin label tempamine. J Magn Reson 163:257–269
Turina P, Petersen J, Gräber P (2016) Thermodynamics of proton transport coupled ATP synthesis. Biochim Biophys Acta 1857:653–664
Ustynyuk LYu, Tikhonov AN (2018) The cytochrome b6f complex: DFT modeling of the first step of plastoquinol oxidation by the iron-sulfur protein. J Organomet Chem 867:290–299
Varco-Merth B, Fromme R, Wang M, Fromme P (2008) Crystallization of the c14-rotor of the chloroplast ATP synthase reveals that it contains pigments. Biochim Biophys Acta 1777:605–612
Vershubskii AV, Tikhonov AN (2020) pH-Dependent regulation of electron and proton transport in chloroplasts in situ and in silico. Biochem (Moscow) Suppl Ser A 14:154–165
Vershubskii AV, Kuvykin IV, Priklonsky VI, Tikhonov AN (2011) Functional and topological aspects of pH-dependent regulation of electron and proton transport in chloroplasts in silico. Biosystems 103:164–179
Vershubskii AV, Trubitsin BV, Priklonskii VI, Tikhonov AN (2017) Lateral heterogeneity of the proton potential along the thylakoid membranes of chloroplasts. Biochim Biophys Acta 1859:388–401
Vershubskii AV, Nevyantsev SM, Tikhonov AN (2018) Modeling of electron and proton transport in chloroplast membranes with regard to thioredoxin-dependent activation of the Calvin-Benson cycle and ATP synthase. Biochem(Moscow) Suppl Ser A 12:287–302
Vlasov AV, Kovalev KV, Marx S-H et al (2019) Unusual features of the c-ring of F1FO ATP synthases. Sci Rep 9(1):1–11
Vollmar M, Schlieper D, Winn M, Büchner C, Groth G (2009) Structure of the c14 rotor ring of the proton translocating chloroplast ATP synthase. J Biol Chem 284:18228–18235
Wada H, Murata N (eds) (2009) Lipids in Photosynthesis: Essential and Regulatory Functions. Springer, Dordrecht
Walker JE (2013) The ATP synthase: the understood, the uncertain and the unknown. Biochem Soc Trans 41:1–16
Wallis JG, Browse J (2002) Mutants of Arabidopsis reveal many roles for membrane lipids. Prog Lipid Res 41:254–278
Witt HT (1979) Energy conversion in the functional membrane of photosynthesis. Analysis by light pulse and electric pulse methods. Biochim Biophys Acta 505:355–427
Yamamoto Y (2016) Quality control of photosystem II: the mechanisms for avoidance and tolerance of light and heat stresses are closely linked to membrane fluidity of the thylakoids. Front Plant Sci 7:1136. https://doi.org/10.3389/fpls.2016.01136
Yamamoto Y, Nishimura M (1976) Characteristics of light-induced H+ transport in spinach chloroplasts at lower temperatures I. Relationship between H+ transport and physical changes of the microenvironment in chloroplast membranes. Plant Cell Physiol 17:11–16
Yamamoto Y, Ford RC, Barber J (1981) Relationship between thylakoid membrane fluidity and the functioning of pea chloroplasts. Effect of cholesteryl hemisuccinate. Plant Physiol 67:1069–1072
Yamori W, Hikosaka K, Way DA (2014) Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation ant temperature adaptation. Photosynth Res 119:101–117
Yan J, Cramer WA (2003) Functional insensitivity of the cytochrome b6f complex to structure changes in the hinge region of the Rieske iron–sulfur protein. J Biol Chem 278:20926–20933
Yan K, Chen P, Shao H, Shao C, Zhao S, Brestic M (2013) Dissection of photosynthetic electron transport process in sweet sorghum under heat stress. PLoS ONE 8(5):e62100
Zaks J, Amarnath K, Kramer DM, Niyogi KK, Fleming GR (2012) A kinetic model of rapidly reversible nonphotochemical quenching. Proc Natl Acad Sci USA 109:15757–15762
Zhou Y, vom Dorp K, Dörman P, Hölzl G (2016) Chloroplast lipids. In: Kirchhoff H (ed) Chloroplasts: current research and future trends. Caister Academic Press, Norfolk, pp 1–24
Zhu XG, Wang Y, Ort DR, Long SP (2013) e-Photosynthesis: a comprehensive dynamic mechanistic model of C3 photosynthesis: from light capture to sucrose synthesis. Plant Cell Environ 36:1711–1727
Acknowledgements
We thank Dr. V.I. Priklonskii for the adjustment of the computer program in order to parameterize the constants of temperature dependences of the partial reactions of electron transport. Experimental studies on bean chloroplasts, used in this work for parametrization of the model parameters, were performed earlier by one of the authors in collaboration with Professor E.K. Ruuge, Professor G.B. Khomutov, and Dr. A.A. Timoshin. We are greatly thankful to all our colleagues and collaborators.
Funding
This work was partly supported by the Russian Foundation for Basic Research (Grant 18-04-00214).
Author information
Authors and Affiliations
Contributions
ANT: design and supervision of the work, data processing, writing of the manuscript. AVV: computer calculations, data processing, discussion of results, preparation of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1: equations and rate constants of the model
Equations (A1–A7) represent the system of ordinary differential equations (ODE), which describe the redox transitions of electron carriers (variables [Fd], [\( {\text{P}}_{ 7 0 0}^{ + } \)], [\( {\text{P}}_{ 6 8 0}^{ + } \)], [Pc], [PQ]), the acidification of the thylakoid lumen ([\( {\text{H}}_{\text{in}}^{ + } \)]), and the yield of ATP (variable [ATP]).
Here, the function \( k_{\text{Q}} ( [ {\text{PQ],[Pc],[H}}_{\text{in}}^{ + } ] ,T ) \) describes the electron transfer from PQH2 to Pc via the Cyt b6f complex [see above Eqs. (1) and (2)]. ΔpH is the trans-thylakoid pH difference, ΔpH = pHout – pHin. We assume that pHout to be constant, pHout = 8, due to sufficiently high buffer capacity of the outer medium. [ADN]0 is the total concentration of ADP and ATP. The model parameters α and β in Eqs. (A6) and (A7) are determined by the rate constants of the proton exchange with the proton-accepting groups of the ATP synthase (see Fig. 3 and the explanations below). Formulating Eqs. (A1–A7), we assume the following stoichiometry between the electron transport and ATP synthase complexes: [PSI]/[PSII]/[b6f]/[CF0-CF1] = 1/1/1/1. The relative capacity of the photo-reducible PQ pool, Fd, and Pc was taken as [PQ]0/[PSI] = 10, [Fd]0/[PSI] = 3, and [Pc]0/[PSI] = 1.5, respectively.
The model parameters L1 and L2 describe the numbers of light quanta per unit time exciting P700 and P680, respectively. Parameter m expresses the stoichiometry of proton transfer through the ATP synthase, H+/ATP; m = n/3 = 14/3 is the stoichiometry ratio, the ratio between a number n = 14 of subunits c in the cn-ring to three ATP molecules formed per one turn (360o) of the membrane rotor cn (Seelert et al. 2000; Vollmar et al. 2009). Constants marked with a subscript “0” are the maximal concentrations of the relevant variables. Constants \( k_{{{\text{P}}_{ 6 8 0} }}^{{}} \), \( k_{{{\text{P}}_{ 7 0 0} }}^{{}} \), kQ, kPc, and kMeh are the effective rate constants of the reactions shown in Fig. 1. Constant kADP governs the effective rate of ATP hydrolysis. The function kQ, which characterizes the oxidation of PQH2, depends on pHin (for details see Eqs. 1 and 2; Dubinskii and Tikhonov 1997; Vershubskii et al. 2011). The model parameters KM and Bin, characterize the buffer properties of the system. Here, KM is the equilibrium constant for the reaction of proton binding by buffer groups inside of thylakoids; the model parameter Bin is the concentration of these buffer groups. In this work, we assume the stoichiometric ratio Bin/PSI = 100 (for details, see Vershubskii et al. 2011). Note that parameters KM and Bin (the left side of Eq. A7) can influence the time-course of the system response to switching the actinic light on. The steady-state levels of all the variables of the model, however, are independent on the KM and Bin values (Dubinskii and Tikhonov 1997).
The formulation of the system of differential equations and the choice of the apparent rate constants have been considered in details in our previous works (Vershubskii et al. 2011, 2018). The rate constants for key stages of electron flow and proton transport were determined by fitting the respective experimental and simulated kinetic curves. In particular, effective rate constant for PQH2 oxidation by the Cyt b6f complex was derived by comparing calculated and experimental plots for the rates of the post-illumination reduction of \( {\text{P}}_{ 7 0 0}^{ + } \) at different pHin ( “Appendix 3”, Fig. 13). Effective rate constants of the transmembrane proton transport coupled to ATP synthesis and passive leak of protons through the thylakoid membrane were determined by the comparison of calculated and experimental data on the light-induced acidification of the thylakoid lumen (for details, see “Appendix 2”; Dubinskii and Tikhonov 1995). The values of the rate constants, which characterize different stages of the electron transfer along the ETC, from the water-splitting complex of PSII to PSI acceptors, were chosen on the basis of the literature data on the kinetics of partial reactions of electron transport in different segments of the chloroplast ETC as described above. The characteristic times of electron transfer reactions are given in Table 1.
Appendix 2: proton transport and ATP synthesis in the model
There are indications that the proton conductivity of a lipid bilayer is determined mainly by the presence of acidic groups in the membrane (Deamer 1987; Gutknecht 1987; Nagle 1987). In this work, the equations for the active (JATP) and passive (Jpass) fluxes of protons were derived from a simple model based on the assumption that the processes of the transmembrane transfer of protons occur through the acidic groups bound either to the ATP synthase or buried inside the thylakoid membrane, respectively (Dubinskii and Tikhonov 1995). According to the model considered in our work, the proton transfer through the CFo segment of the ATP synthase includes stages of protonation and deprotonation of carboxyl groups of the cm-ring: –COO– + \( {\text{H}}_{\text{in}}^{ + } \) → –COOH → –COO– + \( {\text{H}}_{\text{out}}^{ + } \). Concerning the passive trans-thylakoid transport of H+ ions, we assume that the proton first binds to the intramembrane proton-accepting group and then dissociates into the stroma. The efficient rate constants of the proton exchange with the proton-accepting group are indicated in Fig. 3b. The rate constants of the direct and reverse reactions are related by the ratio \( k_{1}^{\text{in}} /k_{ - 1}^{\text{in}} = K_{\text{M1}} \) and \( k_{2}^{\text{out}} /k_{ - 2}^{\text{out}} = K_{\text{M2}} \), where KM1 and KM2 are the effective constants of the proton equilibrium for the buffer group –COO− and hydrogen ions inside and outside of the thylakoid, respectively. It is reasonable to assume that, for the proton-accepting groups –COO− fixed in the membrane and involved in the passive transfer of protons across the membrane, the equality KM1 = KM2 ≡ KM must be true. Fitting of the rate constant parameters related to proton transfer through the membrane acidic groups has been performed by means of the comparison of calculated and experimental data on the light-induced uptake of protons by the chloroplasts (Dubinskii and Tikhonov 1995). For the ΔpH-driven flux of protons through the ATP synthase, JATP, coupled to ATP synthesis, we used the following relationship:
where ΔpH = pHout − pHin is the driving force for the operation of the ATP synthase (for details, see Tikhonov and Vershubskii 2014). Coefficients α and β are the model parameters, the values of which are determined by pKA of the acidic group –COO− of the cn-ring of the ATP synthase and the values of the efficient rate constants k1 and k2 characterizing proton transport to -COO− from the lumen and stroma, respectively; \( \alpha = 10^{ - \text{p}K_\text{A}} \left( {1 + \beta } \right) \), \( \beta = k_{ 2}^{\text{out}} /k_{1}^{\text{in}} \). In our calculations we used the model parameters pKA = 7.3 and β = 20, which values have been chosen on the basis of our previous works (Vershubskii et al. 2011, 2017, 2018; Vershubskii and Tikhonov 2020). Formula (A8) provides a sigmoid dependence of the ATP synthesis rate versus the proton-motive force ΔpH (Fig. 3c), which is typical of experimental force–flux relationships in chloroplasts (Turina et al. 2016). Note that variations of the model parameters α and β influence markedly a threshold ΔpHth, above which value the ATP synthase efficiently produces ATP. Numerical values of the model parameters, related to the trans-thylakoid proton transfer, were determined by fitting theoretical curves to relevant experimental dependences of the light-induced acidification of the lumen at different values of external pHout. Fitting of the rate constants \( k_{ 1}^{\text{in}} \), \( k_{ - 1}^{\text{in}} \), \( k_{ 2}^{\text{in}} \), and \( k_{ - 2}^{\text{in}} \) was performed earlier (Dubinskii and Tikhonov 1995).
The model parameter \( k_{{{\text{H}}^{ + } }} \), which stands in the right side of Eq. A7, determines the passive efflux of protons (Jpass) from the lumen to the outer space: Jpass = \( k_{{{\text{H}}^{ + } }} (T )\cdot ( [ {\text{H}}_{\text{in}}^{ + } ] { } - {\text{ [H}}_{\text{out}}^{ + } ] ) \). The \( k_{{{\text{H}}^{ + } }} (T ) \) values were chosen by means of fitting calculated values of Jpass to experimentally measured proton fluxes determined for chloroplasts in the metabolic state 4 (compare experimental and theoretical data in Fig. 4c).
It is well-known fact that the ATP synthase activity is controlled by the redox status of chloroplasts. The light-induced activation of the ATP synthase is associated with the reduction of the thiol groups in the subunit γ, rotating together with the cn-ring (Bakker-Grunwald and van Dam 1974; Bald et al. 2001). It is conceivable that the reduction of these groups may be mediated through the pigments found in the central cavity of the c14-rotor (Varco-Merth et al. 2008; Vlasov et al. 2019). In the current work, however, we ignored this effect, because we compared experimental and theoretical data on the initial phase of the light-induced ATP synthesis (during 10-s illumination), when the linear growth of ATP concentration was not affected by the light-induced modulation of the chloroplast ATP synthase. According to our previous measurements, the light-induced activation of the ATP synthase was observed after 2 min of chloroplast illumination in the presence of methylviologen (data not shown).
Appendix 3: materials and experimental methods
Plant material and preparation of chloroplasts
Class B chloroplasts were isolated from greenhouse bean leaves (Vicia faba, 2–3 weak old) as described by Tikhonov et al. (1981). Chloroplasts were suspended at a final concentration of 2–3 mg chlorophyll/ml in the medium containing 0.2 M sucrose, 2 mM MgCI2, and 10 mM Tricine-NaOH buffer (pH between 6.5 and 9.5) or Mes-HCl buffer (pH between 4.5 and 6.5). For measurements of chloroplast activity at different pH of the chloroplast suspension, we also used the medium which contained 0.2 M sucrose, 2 mM MgC12, 10 mM phosphate-citrate buffer, and 4 mM Mg-ADP. 20 μM methylviologen was used as a mediator of electron transfer from PSI to molecular oxygen. The pH value of the suspending medium was checked with a Radiometer glass electrode GK2321C.
In this work, we refer to experimental results described in earlier works of our group (Tikhonov et al. 1980, 1981, 1983, 1984; Timoshin et al. 1984; Kukushkin and Tikhonov 1988; Tikhonov and Subczynski 2005). The batches of chloroplasts used in these works were isolated from bean leaves of different harvests, including plants grown under variable experimental conditions (different seasons and concomitant changes in environmental conditions). In general, plants were cultivated at growth temperatures in the range 18–32 °C, depending on the season. We found that variability in the plant cultivation conditions could cause somewhat different temperature dependences of photosynthetic processes in isolated bean chloroplasts (compare, for example, panels c, d, and e in Fig. 9). Variability of this kind proved useful for analyzing the structure–function relationships in chloroplasts. In particular, statistically significant coincidence of the peculiar temperatures of the “structural” (membrane fluidity) and “functional” (ATP synthesis) characteristics was observed for each individual batch of chloroplasts (Fig. 9). Inflexion points in temperature dependences of the plots of “structural” and “functional” parameters coincided with a sufficiently high precision (±1 °C). In the meantime, the harvest-depending scattering of these peculiar points was more significant (for example, in the range 25–33 °C, Fig. 9). This observation allowed us to suggest that the temperature dependences of electron and proton transport processes were controlled by the physical state of thylakoid membranes.
EPR measurements of PSII activity and the intersystem electron transfer
The redox transients of P700 were monitored by measuring the light-induced changes in the amplitude of the EPR signal from \( {\text{P}}_{700}^{ + } \) (Tikhonov et al. 1980, 1981; Tikhonov 2015). The EPR measurements of \( {\text{P}}_{700}^{ + } \) were performed with a Varian EPR spectrometer (model E-4) at 4 G modulation amplitude and 10 mW microwave power. Far-red background illumination (interference filter SIF707, Karl Zeiss Jena; λmax = 707 nm, Δλl/2 = 5 nm) was applied to provide the re-oxidation of the ETC between PSII and PSI. The intensity of this light, provided by a 150 W incandescent lamp equipped with a water filter and focusing lens, was adjusted to reach maximal level of P700 oxidation. A similar light source without the interference filter (white light) was used for efficient excitation of both photosystems. Two kinds of white light pulses of were used to test PSII activity: (i) short flashes (τl/2 = 7 μs) of saturating intensity, and (ii) long flashes (τl/2 = 750 μs) were applied for multiple operation of P680. The energy released in the discharge circuit was 10 and 100 J, respectively (Tikhonov et al. 1980).
Figure 12a shows a simplified diagram illustrating electron transfer from PSII and O2, the terminal acceptor of electrons donated by PSI. Illumination of chloroplasts by the far-red (or white) light induces generation of the EPR signal from \( {\text{P}}_{700}^{ + } \) shown in Fig. 12b. After sudden shutdown of white light (WL), \( {\text{P}}_{700}^{ + } \) rapidly reduces due to electrons donated by reduced PQH2 molecules (Fig. 12c). The half-time of \( {\text{P}}_{700}^{ + } \) decay (parameter τ1/2) characterizes the rate of electron transfer from PQH2 to \( {\text{P}}_{700}^{ + } \).

Modified figures adopted from (Tikhonov et al. 1984)
Simplified diagram illustrating electron transfer from PSII and O2, the terminal acceptor of electrons donated by PSI (panel a); EPR signals of bean chloroplasts in the dark and during illumination with the far-red light, λmax = 707 nm, as indicated (panel b); the post-illumination kinetics of \( {\text{P}}_{700}^{ + } \) reduction in bean chloroplasts pre-illuminated by continuous white light (panel c)
Figure 13 shows experimental and theoretical dependencies of the half-time of \( {\text{P}}_{700}^{ + } \) reduction after switching off the white light on the intra-thylakoid pHin. Open symbols, experimental data; filled symbols, calculated data. Experimental points were obtained for the suspension of uncoupled chloroplasts (on the basis of results published by Tikhonov et al. (1980)).
Experimental and theoretical dependencies of the half-time of \( {\text{P}}_{700}^{ + } \) reduction after switching off the white light on the intra-thylakoid pHin. Open symbols, experimental data; filled symbols, calculated data. Experimental points were obtained for the suspension of uncoupled chloroplasts (on the basis of results published in Tikhonov et al. 1984)
Figure 14a shows the time-course of P700 redox changes in aerated suspension of bean chloroplasts. Illumination of chloroplasts by the far-red light (λ707), absorbed predominantly by PSI, induced oxidation of P700. Application of a short saturating pulse (τ1/2 = 7 µs) induced the reduction of \( {\text{P}}_{700}^{ + } \) due to the injection of electrons from PSII to the intersystem ETC. The reduction of \( {\text{P}}_{700}^{ + } \) is followed by the re-oxidation of P700 due to the action of the continuous far-red light. The area W1 over the kinetic curve can serve as a measure of PSII photochemical activity: in response to a short flash, each PSII donates one electron (on an average). In this case, the Mn4Ca cluster is oxidized by one electron and one electron is donated to the intersystem ETC (Cardona et al. 2012). After the action of a prolonged flash (τ1/2 = 750 µs), the area over the kinetic curve (parameter W2) increases. This occurs due to a multiple charge separations in PSII and donation of several electrons to the PQ pool (Tikhonov and Vershubskii 2017). Figure 14b shows the temperature dependences of parameters W1 and W2. The ratio f = W2/W1 is determined by the rate of electron transfer from PSII to the PQ pool (Fig. 14c). The temperature of a sample was regulated with the Varian temperature controller.
a The light-induced redox transients of P700 in bean chloroplasts induced by the far-red light (λ707) and pulses of white light of different durations. Parameters W1 and W2 are proportional to the numbers of electrons injected into intersystem electron transport chain (for other details see text and Tikhonov et al. 1980). b Temperature dependences of parameters W1 and W2 shown in the panel a. c Temperature dependence of the ratio f = W2(T)/W1(T), which determines a number of electrons donated by PSII during the action of the long flash
Potentiometry methods of assaying the chloroplasts activity
The rate of pseudocyclic electron flow JFd–O2 (H2O → PSII → PSI → MV → O2; the so-called “water-water” cycle; Asada 1999) was determined by measuring the O2 uptake (O2 + e− → O •−2 ) in an aerated suspension of chloroplasts, using a laboratory-made Clark-type electrode. In this case, the superoxide and catalase activities of chloroplasts were inhibited by the addition of small amounts of NaN3 as described earlier (Timoshin et al. 1984).
The rate of ATP formation (VATP) was routinely measured by the potentiometry method (Nishimura et al. 1962; Timoshin et al. 1984). All controls including that of adenylate kinase activity were properly made. Adequacy of potentiometric measurements of photophosphorylation (ADP + Pi → ATP + H2O) was verified independently by the use of enzymatic (Malenkova et al. 1982) and modified luciferin-luciferase (Ataullakhanov and Pichugin 1981) methods. The rate of ATP hydrolysis was also determined by measuring changes in the 31P NMR spectra of ATP, ADP, and Pi in bean chloroplast suspension according to (Ogawa et al. 1980). Results of this study, performed together with professor E.K. Ruuge, will be published elsewhere.
Having measured, under the same experimental conditions, the rates of ATP synthesis (VATP) and electron transport (JFd–O2), we could determine the ratio VATP/JFd–O2, which characterizes the efficiency of coupling of electron transport and ATP synthesis (often termed as the so-called ratio “P/2e”; Ivanov 1993). Figure 15 reproduces the temperature dependence of the ratio P/2e that was determined earlier in our work (Timoshin et al. 1984). As one can see, the ratio P/2e increases with temperature, reaching a plateau at temperatures above 25 °C.

Modified figures after (Timoshin et al. 1984)
Temperature dependences of parameter P/2e, which denotes the ratio between the rates of photophosphorylation (ADP + Pi → ATP) and pseudocyclic electron flow and ATPase activity of bean chloroplasts (closed and open symbols were obtained on different batches of chloroplasts)
Measurements of ΔpH generation
Transmembrane pH difference (ΔpH) across the thylakoid membrane was measured by three independent methods based on the use of the EPR technique: (1) the determination of the pHin-dependent rate of the intersystem electron transfer from the kinetics of the post-illumination reduction of \( {\text{P}}_{700}^{ + } \) (Tikhonov et al. 1981, 1984), (2) the use of pH-sensitive water-soluble spin probes located in the thylakoid lumen (Tikhonov et al. 2008; Tikhonov 2017; Vershubskii et al. 2017), and (3) the determination of ΔpH in chloroplasts from the partitioning of a water-soluble spin label tempamine (Trubitsin and Tikhonov 2003; Tikhonov 2017). We used experimental data on ΔpH measurements in bean chloroplasts in order to perform the final fitting of the model parameters (see, for example, Vershubskii and Tikhonov 2020).
Spin-labeling study of thermo-induced structural changes in thylakoid membranes
Thermo-induced changes in the lipid domains of the thylakoid membranes were studied with the lipid-soluble spin probes, paramagnetic derivatives of stearic acid, 5-SASL (Fig. 9b), as described in Tikhonov et al. (1980, 1983), Timoshin et al. (1984), Lutova and Tikhonov (1988), Ligeza et al. (1998), and Tikhonov and Subczynski (2005). A small aliquot (1% v/v) of 5-SASL dissolved in ethanol was added to chloroplast suspension. After 10-min incubation of thylakoids with the spin probe, the suspension of spin-labeled thylakoids was used for EPR measurements. The temperature of a sample placed into the cavity of a Varian (E-4) X-band EPR spectrometer was regulated with the Varian temperature controller, with precision up to ± 0.5 °C.
Rights and permissions
About this article
Cite this article
Tikhonov, A.N., Vershubskii, A.V. Temperature-dependent regulation of electron transport and ATP synthesis in chloroplasts in vitro and in silico. Photosynth Res 146, 299–329 (2020). https://doi.org/10.1007/s11120-020-00777-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-020-00777-0