Abstract
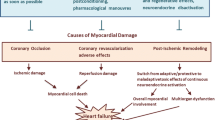
The deiodinases regulate the activation and inactivation of Thyroid hormones (TH), in both physiological and pathological conditions. The three deiodinases, DIO1, DIO2 and DIO3, have different catalytic role and cellular and tissue distribution. Aim of this study is to evaluate a rat model of regional ischemia/reperfusion (I/R), the modification of cardiac main function after the administration of 6 µg/kg/day of triiodothyronine (T3), and the associated to DIO1, DIO2 and DIO3 gene expression. We also aim to study DIO1 and DIO2 protein levels in different left ventricular regions after an ischemic event. Four groups of rats were studied: sham-operated, sham-operated + T3, I/R rats and I/R rats + T3. DIO1, DIO2 and DIO3 expression were evaluated in I/R region (AAR: area-at-risk) and in a more distant region from ischemic wound (RZ: remote zone). In I/R group, circulating free-T3 (FT3) levels were significantly decreased with respect to basal values, whereas in I/R + T3 rats, FT3 levels were comparable to basal values. In AAR of I/R + T3 rats, DIO1 and DIO2 gene expression significantly increased with respect to sham. In RZ, DIO1 and DIO3 gene expression was significantly lower in sham and I/R rats when compared to I/R + T3. In sham + T3 group, DIO1 and DIO2 gene expression was not detectable, whereas DIO3 was significantly higher than in the other three groups. The present study gives interesting new insights on DIO1, DIO2 and DIO3 in the ischemic heart and their role in relation to T3-mediated amelioration of cardiac function and structure.




Similar content being viewed by others
References
Pantos CI, Malliopoulou VA, Mourouzis IS, Karamanoli EP, Paizis IA, Steimberg N, Varonos DD, Cokkinos DV (2004) Long-term thyroxine administration protects the heart in a pattern similar to ischemic preconditioning. Thyroid. https://doi.org/10.1089/10507250252949469
Lee S, Farwell AP (2016) Euthyroid sick syndrome. Compr Physiol 6:1071–1080. https://doi.org/10.1002/cphy.c150017
Sato Y, Yoshihisa A, Kimishima Y, Kiko T, Kanno Y, Yokokawa T, Abe S, Misaka T, Sato T, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Takeishi Y (2019) Low T3 syndrome is associated with high mortality in hospitalized patients with heart failure. J Card Fail 25:195–203. https://doi.org/10.1016/j.cardfail.2019.01.007
Pingitore A, Landi P, Taddei MC, Ripoli A, L'Abbate A, Iervasi G (2005) Triiodothyronine levels for risk stratification of patients with chronic heart failure. Am J Med 118:132–136. https://doi.org/10.1016/j.amjmed.2004.07.052
Rothberger GD, Gadhvi S, Michelakis N, Kumar A, Calixte R, Shapiro LE (2017) Usefulness of serum triiodothyronine (T3) to predict outcomes in patients hospitalized with acute heart failure. Am J Cardiol 119:599–603. https://doi.org/10.1016/j.amjcard.2016.10.045
Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeöld A, Bianco AC (2008) Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev 29:898–938. https://doi.org/10.1210/er.2008-0019
Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR (2002) Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89. https://doi.org/10.1210/edrv.23.1.0455
Toyoda N, Kaptein E, Berry MJ, Harney JW, Larsen PR, Visser TJ (1997) Structure–activity relationships for thyroid hormone deiodination by mammalian type i iodothyronine deiodinases. Endocrinology 138:213–219. https://doi.org/10.1210/endo.138.1.4900
Oppenheimer JH, Schwartz HL, Surks MI (1972) Propylthiouracil inhibits the conversion of L-thyroxine to L-triiodothyronine: an explanation of the antithyroxine effect of propylthiouracil and evidence supporting the concept that triiodothyronine is the active thyroid hormone. J Clin Invest 51:2493–2497. https://doi.org/10.1172/JCI107063
Olivares EL, Marassi MP, Fortunato RS, da Silva ACM, Costa-e-Sousa RH, Araújo IG, Mattos EC, Masuda MO, Mulcahey MA, Huang SA, Bianco AC, Carvalho DP (2007) Thyroid function disturbance and type 3 iodothyronine deiodinase induction after myocardial infarction in Rats-A time course study. Endocrinology 148:4786–4792. https://doi.org/10.1210/en.2007-0043
Pol CJ, Muller A, Simonides WS (2010) Cardiomyocyte-specific inactivation of thyroid hormone in pathologic ventricular hypertrophy: an adaptative response or part of the problem? Heart Fail Rev 15:133–142. https://doi.org/10.1007/s10741-008-9133-7
Pol CJ, Muller A, Zuidwijk MJ, van Deel ED, Kaptein E, Saba A, Marchini M, Zucchi R, Visser TJ, Paulus WJ, Duncker DJ, Simonides WS (2011) Left-ventricular remodeling after myocardial infarction is associated with a cardiomyocyte-specific hypothyroid condition. Endocrinology 152:669–679. https://doi.org/10.1210/en.2010-0431
Boelen A, Maas MA, Lowik CW, Platvoet MC, Wiersinga MW (1996) Induced illness in interleukin-6 (IL-6) knock-out mice: a causal role of IL-6 in the development of the low 3,5,3'-triiodothyronine syndrome. Endocrinology 137:5250–5254. https://doi.org/10.1210/endo.137.12.8940342
Simonides WS, Mulcahey MA, Redout EM, Muller A, Zuidwijk MJ, Visser TJ, Wassen FW, Crescenzi A, da-Silva WS, Harney J, Engel FB, Obregon M-J, Larsen PR, Bianco AC, Huang SA (2008) Hypoxia inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J Clin Invest 118:975–983. https://doi.org/10.1172/JCI32824
Dentice M, Morisco C, Vitale M, Rossi G, Fenzi G, Salvatore D (2003) The different cardiac expression of the type 2 iodothyronine deiodinase gene between human and rat is related to the differential response of the Dio2 genes to Nkx-2.5 and GATA-4 transcription factors. Mol Endocrinol 17:1508–1521. https://doi.org/10.1210/me.2002-0348
Sabatino L, Iervasi G, Ferrazzi P, Francesconi D, Chopra IJ (2000) A study of iodothyronine 5′-monodeiodinase activities in normal and pathological tissues in man and their comparison with activities in rat tissues. Life Sci 68:191–202. https://doi.org/10.1016/s0024-3205(00)00929-2
Sabatino L, Chopra IJ, Tanavoli S, Iacconi P, Iervasi G (2001) A radioimmunoassay for type I iodothyronine 5′-monodeiodinase in human tissues. Thyroid 11:733–739. https://doi.org/10.1089/10507250152484565
Sabatino L, Balzan S, Kusmic C, Iervasi G (2018) Modification of gene expression profiling related to renin-angiotensin system in an ischemia/reperfusion rat model after T3 infusion. Mol Cell Biochem 449:277–283. https://doi.org/10.1007/s11010-018-3364-2
Saba A, Donzelli R, Colligiani D, Raffaelli A, Nannipieri M, Kusmic C, Dos Remedios CG, Simonides WS, Iervasi G, Zucchi R (2014) Quantification of thyroxine and 3,5,3′-triiodo-thyronine in human and animal hearts by a novel liquid chromatography-tandem mass spectrometry method. Horm Metab Res 46:628–634. https://doi.org/10.1055/s-0034-1368717
Ojamaa K, Kenessey A, Shenoy R, Klein I (2000) Thyroid hormone metabolism and cardiac gene expression after acute myocardial infarction in the rat. Am J Physiol Endocrinol Metab 279:E1319–E1324. https://doi.org/10.1152/ajpendo.2000.279.6.E1319
Bianco AC, da Conceição RR (2018) The deiodinase trio and thyroid hormone signaling. Methods Mol Biol 1801:67–83. https://doi.org/10.1007/978-1-4939-7902-8_8
Luongo C, Dentice M, Salvatore D (2019) Deiodinases and their intricate role in thyroid hormone homeostasis. Nat Rev Endocrinol 15:479–488. https://doi.org/10.1038/s41574-019-0218-2
Baqui MM, Gereben B, Harney JW, Larsen PR, Bianco AC (2000) Distinct subcellular localization of transiently expressed types 1 and 2 iodothyronine deiodinases as determined by immunofluorescence confocal microscopy. Endocrinology 141:4309–4312. https://doi.org/10.1210/endo.141.11.7872
Maia AL, Goemann IM, Meyer EL, Wajner SM (2011) Type 1 iodothyronine deiodinase in human physiology and disease. J Endocrinol 209:283–297. https://doi.org/10.1530/JOE-10-0481
Rajagopalan V, Zhang Y, Ojamaa K, Ojamaa K, Chen Y-F, Pingitore A, Pol CJ, Saunders D, Balasubramanian K, Towner RA, Gerdes AM (2016) Safe oral triiodo-L-thyronine therapy protects from post-infarct cardiac dysfunction and arrhythmias without cardiovascular adverse effects. PLoS ONE 11:e0151413. https://doi.org/10.1371/journal.pone.0151413
Gereben B, Goncalves C, Harney JW, Larsen PR, Bianco AC (2000) Selective proteolysis of human type 2 deiodinase: a novel ubiquitin-proteasomal mediated mechanism for regulation of hormone activation. Mol Endocrinol 14:1697–1708. https://doi.org/10.1210/mend.14.11.0558
Steinsapir J, Bianco AC, Buettner C, Harney J, Larsen PR (2000) Substrate-induced down-regulation of human type 2 deiodinase (hD2) is mediated through proteasomal degradation and requires interaction with the enzyme's active center. Endocrinology 141:1127–1135. https://doi.org/10.1210/endo.141.3.7355
Acknowledgements
The authors want to thank Dr.Sohrab Tanavoli for helpful English revision and Dr. Silvana Balzan for useful advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sabatino, L., Kusmic, C. & Iervasi, G. Modification of cardiac thyroid hormone deiodinases expression in an ischemia/reperfusion rat model after T3 infusion. Mol Cell Biochem 475, 205–214 (2020). https://doi.org/10.1007/s11010-020-03873-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03873-w