Abstract
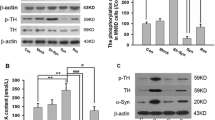
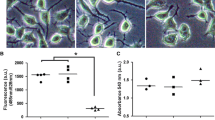
5′-Nucleotidase domain-containing protein 2 (NT5DC2) has been revealed by genome-wide association studies (GWAS) as a gene implicated in neuropsychiatric disorders related to the abnormality of dopamine (DA) activity in the brain. Based on its amino acid sequence, NT5DC2 is assumed to be a member of the family of haloacid dehalogenase-type phosphatases; although there is no information about its function and structural conformation. We recently reported that NT5DC2 binds to tyrosine hydroxylase (TH) and that the down-regulation of NT5DC2 tended to increase DA synthesis. In this study, we investigated whether NT5DC2 could regulate the catalytic activity of TH, which converts tyrosine to DOPA, because the phosphorylation level of TH, controlled by protein kinases and phosphatases, is well known to regulate its catalytic activity. The down-regulation of NT5DC2 by siRNA increased mainly DOPA synthesis by TH in PC12D cells, although this down-regulation tended to increase the conversion of DOPA to DA by aromatic l-amino acid decarboxylase. The increased DOPA synthesis should be attributed to the catalytic activity of TH controlled by its phosphorylation, because Western blot analysis revealed that the down-regulation of NT5DC2 tended to increase the level of TH phosphorylated at its Ser residues, but not that of the TH protein. Moreover, the induction of kinase activity by forskolin markedly potentiated the phosphorylation of TH at its Ser40 in PC12D cells having down-regulated NT5DC2. Immunocytochemical analysis of PC12D cells demonstrated that NT5DC2, TH protein, and TH phosphorylated at its Ser40 were predominantly localized in the cytoplasm and that the localization of NT5DC2 and TH proteins partially overlapped. Collectively, our results indicate that NT5DC2 could work to inhibit the DOPA synthesis by decreasing the phosphorylation of TH at its Ser40. We propose that NT5DC2 might decrease this phosphorylation of TH by promoting dephosphorylation or by inhibiting kinase activity.





Similar content being viewed by others
Abbreviations
- TH:
-
Tyrosine hydroxylase
- AADC:
-
Aromatic amino acid decarboxylase
- DA:
-
Dopamine
- NT5DC2:
-
5′-Nucleotidase domain-containing protein 2
References
Berresheim U, Kuhn DM (1994) Dephosphorylation of tyrosine hydroxylase by brain protein phosphatases: a predominant role for type 2A. Brain Res 637:273–276
Bevilaqua LRM, Graham ME, Dunkley P, Bevilaqua LR, Graham ME, Dunkley PR, von Nagy-Felsobuki EI, Dickson PW (2001) Phosphorylation of Ser(19) alters the conformation of tyrosine hydroxylase to increase the rate of phosphorylation of Ser(40). J Biol Chem 276:40411–40416
Bevilaqua LR, Cammarota M, Dickson PW, Sim AT, Dunkley PR (2003) Role of protein phosphatase 2C from bovine adrenal chromaffin cells in the dephosphorylation of phospho-serine 40 tyrosine hydroxylase. J Neurochem 85:1368–1373
Bobrovskaya L, Cheah TB, Bunn SJ, Dunkley PR (1998) Tyrosine hydroxylase in bovine adrenal chromaffin cells: angiotensin II-stimulated activity and phosphorylation of Ser19, Ser31, and Ser40. J Neurochem 70:2565–2573
Bobrovskaya L, Dunkley PR, Dickson PW (2004) Phosphorylation of Ser19 increases both Ser40 phosphorylation and enzyme activity of tyrosine hydroxylase in intact cells. J Neurochem 90:857–864
Daubner SC, Le T, Wang S (2011) Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys 508:1–12
Duchemin AM, Berry MD, Neff NH, Hadjiconstantinou M (2000) Phosphorylation and activation of brain aromatic l-amino acid decarboxylase by cyclic AMP-dependent protein kinase. J Neurochem 75:725–731
Duchemin AM, Neff NH, Hadjiconstantinou M (2010) Aromatic l-amino acid decarboxylase phosphorylation and activation by PKGI alpha in vitro. J Neurochem 114:542–552
Dunkley PR, Dickson PW (2019) Tyrosine hydroxylase phosphorylation in vivo. J Neurochem 149:706–728
Dunkley PR, Bobrovskaya L, Graham ME, von Nagy-Felsobuki EI, Dickson PW (2004) Tyrosine hydroxylase phosphorylation: regulation and consequences. J Neurochem 91:1025–1043
Fujisawa H, Okuno S (2005) Regulatory mechanism of tyrosine hydroxylase activity. Biochem Biophys Res Commun 338:271–276
Haavik J, Schelling DJ, Campbell DG, Andersson KK, Flatmark T, Cohen P (1989) Identification of protein phosphatase 2A as the major tyrosine hydroxylase phosphatase in adrenal medulla and corpus striatum: evidence from the effects of okadaic acid. FEBS Lett 251:36–42
Hua G, Xiaolei L, Weiwei Y, Hao W, Yuangang Z, Dongmei L, Yazhuo Z, Hui Y (2015) Protein phosphatase 2A is involved in the tyrosine hydroxylase phosphorylation regulated by α-synuclein. Neurochem Res 40:428–437
Ichimura T, Isobe T, Okuyama T, Yamauchi T, Fujisawa H (1987) Brain 14-3-3 protein is an activator protein that activates tryptophan 5-monooxygenase and tyrosine 3-monooxygenase in the presence of Ca2+, calmodulin-dependent protein kinase II. FEBS Lett 219:79–82
Itagaki C, Isobe T, Taoka M, Natsume T, Nomura N, Horigome T, Omata S, Ichinose H, Nagatsu T, Greene LA, Ichimura T (1999) Stimulus-coupled interaction of tyrosine hydroxylase with 14-3-3 proteins. Biochemistry 38:15673–15680
Kansy JW, Daubner SC, Nishi A, Sotogaku N, Lloyd MD, Nguyen C, Lu L, Haycock JW, Hope BT, Fitzpatrick PF, Bibb JA (2004) Identification of tyrosine hydroxylase as a physiological substrate for Cdk5. J Neurochem 91:374–384
Karasawa N, Arai R, Isomura G, Yamada K, Sakai K, Sakai M, Nagatsu T, Nagatsu I (1994) Phenotypic changes of AADC-only immunopositive premammillary neurons in the brain of laboratory shrew Suncus murinus by systemic administration of monoamine precursors. Neurosci Lett 179:65–70
Kleppe R, Toska K, Haavik J (2001) Interaction of phosphorylated tyrosine hydroxylase with 14-3-3 proteins: evidence for a phosphoserine 40-dependent association. J Neurochem 77:1097–1107
Kornilov SA, Rakhlin N, Koposov R, Lee M, Yrigollen C, Caglayan AO, Magnuson JS, Mane S, Chang JT (2016) Grigorenko EL (2016) Genome-wide association and exome sequencing study of language disorder in an isolated population. Pediatrics 137(4):e20152469. https://doi.org/10.1542/peds.2015-2469
Lehmann IT, Bobrovskaya L, Gordon SL, Dunkley PR, Dickson PW (2006) Differential regulation of the human tyrosine hydroxylase isoforms via hierarchical phosphorylation. J Biol Chem 281:17644–17651
Li XM, Qi J, Juorio AV, Boulton AA (1997) Reciprocal regulation of the content of aromatic l-amino acid decarboxylase and tyrosine hydroxylase mRNA by NGF in PC12 cells. J Neurosci Res 47:449–454
Nagatsu T, Levitt M, Udenfriend S (1964) Tyrosine hydroxylase: the initial step in norepinephrine biosynthesis. J Biol Chem 239:2910–2917
Nagatsu I, Sakai M, Yoshida M, Nagatsu T (1988) Aromatic l-amino acid decarboxylase-immunoreactive neurons in and around the cerebrospinal fluid-contacting neurons of the central canal do not contain dopamine or serotonin in the mouse and rat spinal cord. Brain Res 475:91–102
Nakashima A, Mori K, Kaneko YS, Hayashi N, Nagatsu T, Ota A (2011) Phosphorylation of the N-terminal portion of tyrosine hydroxylase triggers proteasomal digestion of the enzyme. Biochem Biophys Res Commun 407:343–347
Nakashima A, Ohnuma S, Kodani Y, Kaneko YS, Nagasaki H, Nagatsu T, Ota A (2016) Inhibition of deubiquitinating activity of USP14 decreases tyrosine hydroxylase phosphorylated at Ser19 in PC12D cells. Biochem Biophys Res Commun 472:598–602
Nakashima A, Yamaguchi H, Kodani Y, Kaneko YS, Kawata M, Nagasaki H, Nagatsu T, Ota A (2019) Identification by nano-LC–MS/MS of NT5DC2 as a protein binding to tyrosine hydroxylase: down-regulation of NT5DC2 by siRNA increases catecholamine synthesis in PC12D cells. Biochem Biophys Res Commun 516:1060–1065
Ong LK, Sominsky L, Dickson PW, Hodgson DM, Dunkley PR (2012) The sustained phase of tyrosine hydroxylase activation in vivo. Neurochem Res 37:1938–1943
Peng X, Tehranian R, Dietrich P, Stefanis L, Perez RG (2005) Alpha-synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J Cell Sci 118:3523–3530
Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ (2002) A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci 22:3090–3099
Prados J, Stenz L, Courtet P, Prada P, Nicastro R, Adouan W, Guillaume S, Olié E, Aubry JM, Dayer A, Perroud N (2015) Borderline personality disorder and childhood maltreatment: a genome-wide methylation analysis. Genes Brain Behav 14:177–188
Ripke S, O’Dushlaine C, Chambert K, Moran JL, Kahler AK, Akterin S, Bergen SE et al (2013) Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet 45:1150–1159
Seifried A, Schultz J, Gohla A (2013) Human HAD phosphatases: structure, mechanism, and roles in health and disease. FEBS J 280:549–571
Tehranian R, Montoya SE, Van Laar AD, Hastings TG, Perez RG (2006) Alpha-synuclein inhibits aromatic amino acid decarboxylase activity in dopaminergic cells. J Neurochem 99:1188–1196
Toska K, Kleppe R, Armstrong CG, Morrice NA, Cohen P, Haavik J (2002) Regulation of tyrosine hydroxylase by stress-activated protein kinases. J Neurochem 83:775–783
van Hulzen KJE, Scholz CJ, Franke B, Ripke S, Klein M, McQuillin A et al (2017) Genetic overlap between attention-deficit/hyperactivity disorder and bipolar disorder: evidence from genome-wide association study meta-analysis. Biol Psychiatry 82:634–641
Wang J, Lou H, Pedersen CJ, Smith AD, Perez RG (2009) 14-3-3zeta contributes to tyrosine hydroxylase activity in MN9D cells: localization of dopamine regulatory proteins to mitochondria. J Biol Chem 284:14011–14019
Young EA, Neff NH, Hadjiconstantinou M (1993) Evidence for cyclic AMP-mediated increase of aromatic 1-amino acid decarboxylase activity in the striatum and midbrain. J Neurochem 60:2331–2333
Zhang D, Kanthasamy A, Yang Y, Anantharam V (2007) Protein kinase C delta negatively regulates tyrosine hydroxylase activity and dopamine synthesis by enhancing protein phosphatase-2A activity in dopaminergic neurons. J Neurosci 27:5349–5362
Zhu M, Juorio A, Paterson I, Boulton A (1992) Regulation of aromatic 1-amino acid decarboxylase by dopamine receptors in the rat brain. J Neurochem 58:636–641
Zhu M, Juorio A, Paterson I, Boulton A (1993) Regulation of striatal aromatic 1-amino acid decarboxylase: effects of blockade or activation of dopamine receptors. Eur J Pharmacol 238:157–164
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number 18K11004 to A. Nakashima.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nakashima, A., Yamaguchi, H., Kondo, M. et al. NT5DC2 affects the phosphorylation of tyrosine hydroxylase regulating its catalytic activity. J Neural Transm 127, 1631–1640 (2020). https://doi.org/10.1007/s00702-020-02236-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-020-02236-5