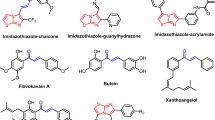
Abstract
Some of the chalcones and flavones are very efficient to facilitate the signal of death to the lung cancer cell through blocking the active site BH3 of Bcl-2 proteins. We designed a number of novel chalcones and flavones targeting the active site of the protein. Docking study showed that the designed compounds were very efficient as the reported compounds. We synthesized and characterized some of the designed compounds. 2,3-Tetrasubstituted-2,3-dihydrobenzofuran-3-carboxamides were generated as novel products by base-catalyzed condensation of o-hydroxyacetophenones and p-nitrobenzaldehyde in different alcohols. Detailed NMR spectroscopic analysis including COSY, homodecoupling and HETCOR (one bond as well as long-range) studies established the structure of the compounds. Mechanistic paths have been suggested for the formation of the novel products.
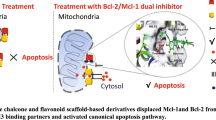
Graphic Abstract
SYNOPSIS: With the help of structural features of naturally occurring anti-cancer chalcones and flavones, we developed a strategy for the synthesis of some novel chalcones and flavones suitable for inhibiting lung cancer. To check their efficiency for this cancer, we have taken help of theoretical calculations. From docking of the reported and designed compounds with the protein targeted by reported compounds, the synthesis of the designed compounds were encouraged us. We synthesized and characterized a number of designed compounds through the simple synthetic method.






Similar content being viewed by others
References
Galati G and O’Brien P J 2004 Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties Free Radic. Biol. Med. 37 287
Wu T C, Yang Y C, Huang P R, Wen Y D and Yeh S L 2012 Genistein enhances the effect of trichostatin A on inhibition of A549 cell growth by increasing expression of TNF receptor-1 Toxicol. Appl. Pharmacol. 262 247
El-Gamal A A, Al-Massarani S M, Abdel-Mageed W M, El-Shaibany A, Al-Mahbashi H M, Basudan O A, Badria F A, Al-Said M S and Abdel-Kader M S 2016 New cytotoxic prenylated flavonoids from Commiphora opobalsamum stem bark Planta Med. 81 S1
Mahapatra D K, Bharti S K and Asati V 2015 Anti-cancer chalcones: Structural and molecular target perspectives Eur. J. Med. Chem. 98 69
Orlikova B, Tasdemir D, Golais F, Dicato M and Diederich M 2011 Dietary chalcones with chemopreventive and chemotherapeutic potential Genes Nutr. 6 125
Singh P, Anand A and Kumar V 2014 Recent developments in biological activities of chalcones: a mini review Eur. J. Med. Chem. 85 758
Mirzaei H and Emami S 2016 Recent advances of cytotoxic chalconoids targeting tubulin polymerization: Synthesis and biological activity Eur. J. Med. Chem. 121 610
Qiu H Y, Wang F, Wang X, Sun W X, Qi J L, Pang Y J, Yang R W, Lu G H, Wang X M and Yang Y H 2017 Design, synthesis, and biological evaluation of chalcone-containing shikonin derivatives as inhibitors of tubulin polymerization Chem. Med. Chem. 12 399
Wang Y, Hedblom A, Koerner S K, Li M, Jernigan F E, Wegiel B and Sun L 2016 Novel synthetic chalcones induce apoptosis in the A549 non-small cell lung cancer cells harboring a KRAS mutation Bioorg. Med. Chem. Lett. 26 5703
Chen G, Zhou D, Li X-Z, Jiang Z, Tan C, Wei X-Y, Ling J, Jing J, Liu F and Li N 2017 A natural chalcone induces apoptosis in lung cancer cells: 3D-QSAR, docking and an in vivo/vitro assay Sci. Rep. 7 10729
Cunha G M, Fontenele J B, Nobre Júnior H V, De Sousa F C, Silveira E R, Nogueira N A, De Moraes M O, Viana G S and Costa-Lotufo L V 2003 Cytotoxic activity of chalcones isolated from lonchocarpus sericeus (pocr.) kunth Phytother Res. 17 155
Hamdy R, Ziedan N I, Aliac S, Bordoni C, El-Sadek M, Lashin E, Brancale A, Jones A T and Westwell A D 2017 Synthesis and evaluation of 5-(1H-indol-3-yl)-N-aryl-1,3,4-oxadiazol-2-amines as Bcl-2 inhibitory anticancer agents Bioorg. Med. Chem. Lett. 27 1037
Ziedan N I, Hamdy R, Cavaliere A, Kourti M, Prencipe F, Brancale A, Jones A T and Westwell A D 2017 A novel mitochondria‐targeting fluorescent probe for hydrogen sulfide in living cells Chem. Biol. Drug. Des. 90 167
Mallik A K, Saha M M, Mallik U K, Goswami S, McPhail D R and McPhail A T 1992 Unexpected synthesis of trans-2,3-dimethoxy-3-(p-formylphenylamino)-4′-nitroflavanones J. Chem. Soc., Chem. Commun. 1992 130
Dhara M G, Mallik U K and Mallik A K 1996 Alkali-catalysed condensation of flavanones and aromatic aldehydes: synthesis of E-3-arylideneflavanones and related compounds Indian J. Chem. 35B 1214
Mallik A K, Dey S P, Chattopadhyay F and Patra A 2002 Novel formation of 6-acyl-5-(2-pyrrolyl)-3H-pyrrolizines by base-catalysed condensation of pyrrole-2-aldehyde with methyl ketones Tetrahedron Lett. 43 1295
Khon W and Sham L J 1965 Self-Consistent Equations Including Exchange and Correlation Effects Phys. Rev. 140 A1133
Sepay N, Mallik S, Guha C and Mallik A K 2016 An efficient synthesis of 1,3-dimethyl-5-(2-phenyl-4H-chromen-4-ylidene) pyrimidine-2,4,6(1H,3H,5H)-triones and investigation of their interactions with β-lactoglobiulin RSC Adv. 6 96016
Sepay N, Guha C, Maity S and Mallik A K 2017 Synthesis of 6,12‐methanobenzo[d]pyrano[3,4‐g][1,3]dioxocin‐1(12H)‐ones and study of their interaction with DNA and β‐lactoglobulin Eur. J. Org. Chem. 2017 6013
Acknowledgements
Financial assistance to S. P. Dey from the University Grants Commission, New Delhi for this work is gratefully acknowledged. We are also grateful to the University of Calcutta and Jadavpur University for providing the instrumental facilities.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Information (SI)
Supplementary Information (SI)
NMR data and figures of the compounds and the docking results with different poses of the synthesized compounds with Bcl-2 protein are available at www.ias.ac.in/chemsci.
Rights and permissions
About this article
Cite this article
Dey, S.P., Sepay, N., Mallik, A.K. et al. Novel chalcones as Bcl-2 inhibitor in lung cancer: docking, design and synthesis of 2,3-Tetrasubstituted-2,3-dihydrobenzofuran-3-carboxamides. J Chem Sci 132, 105 (2020). https://doi.org/10.1007/s12039-020-01812-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-020-01812-2