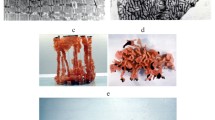
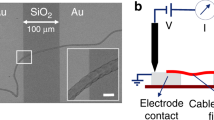
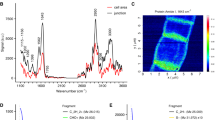
Abstract
The concept of “electric cables” involved in bioenergetic processes of a living cell was proposed half a century ago [Skulachev, V. P. (1971) Curr. Top. Bioenerg., Elsevier, pp. 127-190]. For many decades, only cell membrane structures have been considered as probable pathways for the electric current, namely, for the transfer of transmembrane electrochemical potential. However, the last ten to fifteen years have brought the discovery of bacterial “electric cables” of a new type. In 2005, “nanowires” conducting electric current over distances of tens of micrometers were discovered in metal- and sulphate-reducing bacteria [Reguera, G. et al. (2005) Nature, 435, pp. 1098-1101]. The next five years have witnessed the discovery of microbial electric currents over centimeter distances [Nielsen, L. P. et al. (2010) Nature, 463, 1071-1074]. This new group of bacteria allowing electric currents to flow over macroscopic distances was later called cable bacteria. Nanowires and conductive structures of cable bacteria serve to solve a special problem of membrane bioenergetics: they connect two redox half-reactions. In other words, unlike membrane “cables”, their function is electron transfer in the course of oxidative phosphorylation for the generation of membrane energy rather than of the end-product. The most surprising is the protein nature of these cables (at least of some of them) indicated by recent data, since no protein wires for the long-distance electron transport had been previously known in living systems.




Similar content being viewed by others
Abbreviations
- ΔµH+ :
-
transmembrane proton electrochemical potential
- ETC:
-
electron transport chain
- MLC:
-
metal-like conductivity
- SEC:
-
superexchange conductivity
REFERENCES
Skulachev, V. P. (1971) Energy transformations in the respiratory chain, Curr. Top. Bioenerg., Elsevier, pp. 127-190.
Skulachev, V. P. (1980) Integrating functions of biomebranes. Problems of lateral transport of energy, metabolites and electrons, Biochim. Biophys. Acta, 604, 297-320.
Ptushenko, V. V. (2020) Electric cables of living cells. I. Energy transfer along coupling membranes, Biochemistry (Moscow), 85, 820-832, doi: https://doi.org/10.1134/S000629792007010X.
Minagawa, J. (2016) A supercomplex of cytochrome bf and photosystem I for cyclic electron flow, in Cytochrome Complexes: Evolution, Structures, Energy Transduction, and Signaling (Cramer, W. A., and Kallas, T., eds) Springer, pp. 453-462.
Dumas, L., Chazaux, M., Peltier, G., Johnson, X., and Alric, J. (2016) Cytochrome b 6 f function and localization, phosphorylation state of thylakoid membrane proteins and consequences on cyclic electron flow, Photosynth. Res., 129, 307-320.
Ptushenko, V. V., Cherepanov, D. A., Krishtalik, L. I., and Semenov, A. Y. (2008) Semicontinuum electrostatic calculations of redox potentials in photosystem I, Photosynth. Res., 97, 55-74.
Ptushenko, V. V., and Krishtalik, L. I. (2018) Reorganization energies of the electron transfer reactions involving quinones in the reaction center of Rhodobacter sphaeroides, Photosynth. Res., 138, 167-175.
Gralnick, J. A., and Newman, D. K. (2007) Extracellular respiration, Mol. Microbiol., 65, 1-11.
Turick, C. E., Tisa, L. S., and Caccavo, F., Jr. (2002) Melanin production and use as a soluble electron shuttle for Fe (III) oxide reduction and as a terminal electron acceptor by Shewanella algae BrY, Appl. Environ. Microbiol., 68, 2436-2444.
Reguera, G., McCarthy, K. D., Mehta, T., Nicoll, J. S., Tuominen, M. T., and Lovley, D. R. (2005) Extracellular electron transfer via microbial nanowires, Nature, 435, 1098-1101.
Nielsen, L. P., Risgaard-Petersen, N., Fossing, H., Christensen, P. B., and Sayama, M. (2010) Electric currents couple spatially separated biogeochemical processes in marine sediment, Nature, 463, 1071-1074.
Pfeffer, C., Larsen, S., Song, J., Dong, M., Besenbacher, F., Meyer, R. L., Kjeldsen, K. U., Schreiber, L., Gorby, Y. A., El-Naggar, M. Y., Leung, K. M., Schramm, A., Risgaard-Petersen, N., and Nielsen, L. P. (2012) Filamentous bacteria transport electrons over centimetre distances, Nature, 491, 218.
Schauer, R., Risgaard-Petersen, N., Kjeldsen, K. U., Bjerg, J. J. T., Jørgensen, B. B., Schramm, A., and Nielsen, L. P. (2014) Succession of cable bacteria and electric currents in marine sediment, ISME J., 8, 1314-1322.
Gorby, Y. A., Yanina, S., McLean, J. S., Rosso, K. M., Moyles, D., Dohnalkova, A., Beveridge, T. J., Chang, I. S., Kim, B. H., Kim, K. S., Culley, D. E., Reed, S. B., Romine, M. F., Saffarini, D. A., Hill, E. A., Shi, L., Elias, D. A., Kennedy, D. W., Pinchuk, G., Watanabe, K., Ishii, S., Logan, B., Nealson, K. H., and Fredrickson, J. K. (2006) Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms, Proc. Natl. Acad. Sci. USA, 103, 11358-11363.
Filman, D. J., Marino, S. F., Ward, J. E., Yang, L., Mester, Z., Bullitt, E., Lovley, D. R., and Strauss, M. (2019) Cryo-EM reveals the structural basis of long-range electron transport in a cytochrome-based bacterial nanowire, Commun. Biol., 2, 219, doi: https://doi.org/10.1038/s42003-019-0448-9.
Tan, Y., Adhikari, R. Y., Malvankar, N. S., Ward, J. E., Woodard, T. L., Nevin, K. P., and Lovley, D. R. (2017) Expressing the Geobacter metallireducens PilA in Geobacter sulfurreducens yields pili with exceptional conductivity, mBio, 8, e02203-6, doi: https://doi.org/10.1128/mBio.02203-16.
Adhikari, R. Y., Malvankar, N. S., Tuominen, M. T., and Lovley, D. R. (2016) Conductivity of individual Geobacter pili, RSC Adv., 6, 8354-8357.
Lampa-Pastirk, S., Veazey, J. P., Walsh, K. A., Feliciano, G. T., Steidl, R. J., Tessmer, S. H., and Reguera, G. (2016) Thermally activated charge transport in microbial protein nanowires, Sci. Rep., 6, 23517, doi: https://doi.org/10.1038/srep23517.
Potapova, T. V., and Koksharova, O. A. (2020) Filamentous cyanobacteria as a prototype of multicellular organisms, Russ. J. Plant Physiol., 67, 17-30.
Tan, Y., Adhikari, R. Y., Malvankar, N. S., Pi, S., Ward, J. E., Woodard, T. L., Nevin, K. P., Xia, Q., Tuominen, M. T., and Lovley, D. R. (2016) Synthetic biological protein nanowires with high conductivity, Small, 12, 4481-4485.
El-Naggar, M. Y., Wanger, G., Leung, K. M., Yuzvinsky, T. D., Southam, G., Yang, J., Lau, W. M., Nealson, K. H., and Gorby, Y. A. (2010) Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1, Proc. Natl. Acad. Sci. USA, 107, 18127-18131.
Sato, M., and Mooney, H. M. (1960) The electrochemical mechanism of sulfide self-potentials, Geophysics, 25, 226-249.
Bigalke, J., and Grabner, E. W. (1997) The geobattery model – a contribution to large scale electrochemistry, Electrochimica Acta, 42, 3443-3452.
Naudet, V., and Revil, A. (2005) A sandbox experiment to investigate bacteria-mediated redox processes on self-potential signals, Geophys. Res. Lett., 32, doi: https://doi.org/10.1029/2005GL022735.
Dueholm, M. S., Larsen, S., Risgaard-Petersen, N., Nierychlo, M., Schmid, M., Bøggild, A., van de Vossenberg, J., Geelhoed, J. S., Meysman, F. J. R., Wagner, M., Nielsen, P. H., Nielsen, L. P., and Schramm, A. (2019) On the evolution and physiology of cable bacteria, Proc. Natl. Acad. Sci. USA, 116, 19116-19125.
Cornelissen, R., Bøggild, A., Thiruvallur Eachambadi, R., Koning, R. I., Kremer, A., Hidalgo-Martinez, S., Zetsche, E.-M., Damgaard, L. R., Bonné, R., Drijkoningen, J., Geelhoed, J. S., Boesen, T., Boschker, H. T. S., Valcke, R., Nielsen, L. P., D’Haen, J., Manca, J. V., and Meysman, F. J. R. (2018) The cell envelope structure of cable bacteria, Front. Microbiol., 9, 3044.
Meysman, F. J. R., Cornelissen, R., Trashin, S., Bonné, R., Martinez, S. H., van der Veen, J., Blom, C. J., Karman, C., Hou, J.-L., Thiruvallur Eachambadi, R., Geelhoed, J. S., De Wael, K., Beaumont, H. J. E., Bart Cleuren, B., Valcke, R., van der Zant, H. S. J., Boschker, H. T. S., and Manca, J. V. (2019) A highly conductive fibre network enables centimetre-scale electron transport in multicellular cable bacteria, Nat.Commun., 10, 1-8.
Bonné, R., Hou, J.-L., Hustings, J., Meert, M., Hidalgo-Martinez, S., Cornelissen, R., D’Haen, J., Thijs, S., Vangronsveld, J., Valcke, R., Cleuren, B., Meysman, F. J. R., and Manca, J. V. (2019) Cable bacteria as long-range biological semiconductors, arXiv Preprint: 191206224.
Trojan, D., Schreiber, L., Bjerg, J. T., Bøggild, A., Yang, T., Kjeldsen, K. U., and Schramm, A. (2016) A taxonomic framework for cable bacteria and proposal of the candidate genera Electrothrix and Electronema, Syst. Appl. Microbiol., 39, 297-306.
Parge, H. E., Forest, K. T., Hickey, M. J., Christensen, D. A., Getzoff, E. D., and Tainer, J. A. (1995) Structure of the fibre-forming protein pilin at 2.6 Å resolution, Nature, 378, 32-38.
Craig, L., Taylor, R. K., Pique, M. E., Adair, B. D., Arvai, A. S., Singh, M., Lloyd, S. J., Shin, D. S., Getzoff, E. D., Yeager, M., Forest, K. T., and Tainer, J. A. (2003) Type IV pilin structure and assembly: X-ray and EM analyses of Vibrio cholerae toxin-coregulated pilus and Pseudomonas aeruginosa PAK pilin, Mol. Cell, 11, 1139-1150.
Mehta, T., Coppi, M. V., Childers, S. E., and Lovley, D. R. (2005) Outer membrane c-type cytochromes required for Fe (III) and Mn (IV) oxide reduction in Geobacter sulfurreducens, Appl. Environ. Microbiol., 71, 8634-8641.
Leang, C., Qian, X., Mester, T., and Lovley, D. R. (2010) Alignment of the c-type cytochrome OmcS along pili of Geobacter sulfurreducens, Appl. Environ. Microbiol., 76, 4080-4084.
Inoue, K., Leang, C., Franks, A. E., Woodard, T. L., Nevin, K. P., and Lovley, D. R. (2011) Specific localization of the c-type cytochrome OmcZ at the anode surface in current-producing biofilms of Geobacter sulfurreducens, Environ. Microbiol. Rep., 3, 211-217.
Qian, X., Reguera, G., Mester, T., and Lovley, D. R. (2007) Evidence that OmcB and OmpB of Geobacter sulfurreducens are outer membrane surface proteins, FEMS Microbiol. Lett., 277, 21-27.
Shi, L., Richardson, D. J., Wang, Z., Kerisit, S. N., Rosso, K. M., Zachara, J. M., and Fredrickson, J. K. (2009) The roles of outer membrane cytochromes of Shewanella and Geobacter in extracellular electron transfer, Environ. Microbiol. Rep., 1, 220-227.
Pirbadian, S., Barchinger, S. E., Leung, K. M., Byun, H. S., Jangir, Y., Bouhenni, R. A., Reed, S. B., Romine, M. F., Saffarini, D. A., Shi, L., Gorby, Y. A., Golbeck, J. H., and El-Naggar, M. Y. (2014) Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components, Proc. Natl. Acad. Sci. USA, 111, 12883-12888.
Malvankar, N. S., Vargas, M., Nevin, K. P., Franks, A. E., Leang, C., Kim, B.-C., Inoue, K., Mester, T., Covalla, S. F., Johnson, J. P., Rotello, V. M., Tuominen, M. T., and Lovley, D. R. (2011) Tunable metallic-like conductivity in microbial nanowire networks, Nat. Nanotechnol., 6, 573-579.
Strycharz-Glaven, S. M., Snider, R. M., Guiseppi-Elie, A., and Tender, L. M. (2011) On the electrical conductivity of microbial nanowires and biofilms, Energy Environ. Sci., 4, 4366-4379.
Boesen, T., and Nielsen, L. P. (2013) Molecular dissection of bacterial nanowires, mBio, 4, e00270-13, doi: https://doi.org/10.1128/mBio.00270-13.
Vargas, M., Malvankar, N. S., Tremblay, P.-L., Leang, C., Smith, J. A., Patel, P., Snoeyenbos-West, O., Nevin, K. P., and Lovley, D. R. (2013) Aromatic amino acids required for pili conductivity and long-range extracellular electron transport in Geobacter sulfurreducens, mBio, 4, e00105-13, doi: https://doi.org/10.1128/mBio.00105-13.
Cosert, K. M., Castro-Forero, A., Steidl, R. J., Worden, R. M., and Reguera, G. (2019) Bottom-up fabrication of protein nanowires via controlled self-assembly of recombinant Geobacter pilins, mBio, 10, e02721-19, doi: https://doi.org/10.1128/mBio.02721-19.
Holmes, D. E., Chaudhuri, S. K., Nevin, K. P., Mehta, T., Methé, B. A., Liu, A., Ward, J. E., Woodard, T. L., Webster, J., and Lovley, D. R. (2006) Microarray and genetic analysis of electron transfer to electrodes in Geobacter sulfurreducens, Environ. Microbiol., 8, 1805-1815.
Malvankar, N. S., Tuominen, M. T., and Lovley, D. R. (2012) Lack of cytochrome involvement in long-range electron transport through conductive biofilms and nanowires of Geobacter sulfurreducens, Energy Environ. Sci., 5, 8651-8659.
Polizzi, N. F., Skourtis, S. S., and Beratan, D. N. (2012) Physical constraints on charge transport through bacterial nanowires, Faraday Discuss., 155, 43-61.
Wang, F., Gu, Y., O’Brien, J. P., Sophia, M. Y., Yalcin, S. E., Srikanth, V., Shen, C., Vu, D., Ing, N. L., Hochbaum, A. I., Egelman, E. H., and Malvankar, N. S. (2019) Structure of microbial nanowires reveals stacked hemes that transport electrons over micrometers, Cell, 177, 361-369.
Bjerg, J. T., Boschker, H. T. S., Larsen, S., Berry, D., Schmid, M., Millo, D., Tataru, P., Meysman, F. J. R., Wagner, M., Nielsen, L. P., and Schramm, A. (2018) Long-distance electron transport in individual, living cable bacteria, Proc. Natl. Acad. Sci. USA, 115, 5786-5791.
Chailakhyan, L. M., Glagolev, A. N., Glagoleva, T. N., Murvanidze, G. V., Potapova, T. V., and Skulachev, V. P. (1982) Intercellular power transmission along trichomes of cyanobacteria, Biochim. Biophys. Acta, 679, 60-67.
Ishii, S., Kosaka, T., Hori, K., Hotta, Y., and Watanabe, K. (2005) Coaggregation facilitates interspecies hydrogen transfer between Pelotomaculum thermopropionicum and Methanothermobacter thermautotrophicus, Appl. Environ. Microbiol., 71, 7838-7845.
Summers, Z. M., Fogarty, H. E., Leang, C., Franks, A. E., Malvankar, N. S., and Lovley, D. R. (2010) Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria, Science, 330, 1413-1415.
Acknowledgements
The author is grateful to Dr. F. O. Kasparinsky, Dr. D. R. Lovley, Dr. J. V. Manca, Dr. J. Oemig, and Dr. M. Strauss for provided figures and to anonymous reviewers for valuable comments that allowed improving the work.
Funding
This study was supported by the Lomonosov Moscow State University (state program no. AAAA-A17-117120570011-4) and by the Emanuel Institute of Biochemical Physics of Russian Academy of Sciences (state program no. 01201253314).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares no conflict of interest in financial or any other sphere. This article does not contain description of studies with human participants or animals performed by the author.
Rights and permissions
About this article
Cite this article
Ptushenko, V.V. Electric Cables of Living Cells. II. Bacterial Electron Conductors. Biochemistry Moscow 85, 955–965 (2020). https://doi.org/10.1134/S0006297920080118
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297920080118