Abstract
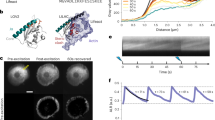
The actin cytoskeleton plays multiple critical roles in cells, from cell migration to organelle dynamics. The small and transient actin structures regulating organelle dynamics are challenging to detect with fluorescence microscopy, making it difficult to determine whether actin filaments are directly associated with specific membranes. To address these limitations, we developed fluorescent-protein-tagged actin nanobodies, termed ‘actin chromobodies’ (ACs), targeted to organelle membranes to enable high-resolution imaging of sub-organellar actin dynamics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The original source data for quantification and the raw imaging data used for all the presented figures and videos are available from https://doi.org/10.5281/zenodo.2851619. Source data are provided with this paper.
Change history
14 August 2020
In the version of this article initially published online, the Zenodo link in the Data Availability section contained a spurious trailing slash that kept it from working. The error has been corrected in the print, PDF and HTML versions of the article.
References
Rocchetti, A., Hawes, C. & Kriechbaumer, V. Fluorescent labelling of the actin cytoskeleton in plants using a cameloid antibody. Plant Methods 10, 12 (2014).
Melak, M., Plessner, M. & Grosse, R. Actin visualization at a glance. J. Cell Sci. 130, 525–530 (2017).
Rapaport, D. Finding the right organelle. Targeting signals in mitochondrial outer-membrane proteins. EMBO Rep. 4, 948–952 (2003).
Riedl, J. et al. Lifeact: a versatile marker to visualize F-actin. Nat. Methods 5, 605–607 (2008).
Sprague, B. L. & McNally, J. G. FRAP analysis of binding: proper and fitting. Trends cell Biol. 15, 84–91 (2005).
Sprague, B. L., Pego, R. L., Stavreva, D. A. & McNally, J. G. Analysis of binding reactions by fluorescence recovery after photobleaching. Biophys. J. 86, 3473–3495 (2004).
Panza, P., Maier, J., Schmees, C., Rothbauer, U. & Sollner, C. Live imaging of endogenous protein dynamics in zebrafish using chromobodies. Development 142, 1879–1884 (2015).
Moore, A. S., Wong, Y. C., Simpson, C. L. & Holzbaur, E. L. Dynamic actin cycling through mitochondrial subpopulations locally regulates the fission–fusion balance within mitochondrial networks. Nat. Commun. 7, 12886 (2016).
Manor, U. et al. A mitochondria-anchored isoform of the actin-nucleating spire protein regulates mitochondrial division. eLife 4, e08828 (2015).
Chakrabarti, R. et al. INF2-mediated actin polymerization at the ER stimulates mitochondrial calcium uptake, inner membrane constriction and division. J. Cell Biol. 217, 251–268 (2018).
Korobova, F., Ramabhadran, V. & Higgs, H. N. An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science 339, 464–467 (2013).
Korobova, F., Gauvin, T. J. & Higgs, H. N. A role for myosin II in mammalian mitochondrial fission. Curr. Biol. 24, 409–414 (2014).
Yang, C. & Svitkina, T. M. Ultrastructure and dynamics of the actin–myosin II cytoskeleton during mitochondrial fission. Nat. Cell Biol. 21, 603–613 (2019).
De Vos, K. J., Allan, V. J., Grierson, A. J. & Sheetz, M. P. Mitochondrial function and actin regulate dynamin-related protein 1-dependent mitochondrial fission. Curr. Biol. 15, 678–683 (2005).
Li, S. et al. Transient assembly of F-actin on the outer mitochondrial membrane contributes to mitochondrial fission. J. Cell Biol. 208, 109–123 (2015).
Ji, W. K., Hatch, A. L., Merrill, R. A., Strack, S. & Higgs, H. N. Actin filaments target the oligomeric maturation of the dynamin GTPase Drp1 to mitochondrial fission sites. eLife 4, e11553 (2015).
Shirai, Y. M. et al. Cortical actin nodes: their dynamics and recruitment of podosomal proteins as revealed by super-resolution and single-molecule microscopy. PLoS ONE 12, e0188778 (2017).
Bisaria, A., Hayer, A., Garbett, D., Cohen, D. & Meyer, T. Membrane-proximal F-actin restricts local membrane protrusions and directs cell migration. Science 368, 1205–1210 (2020).
Lam, S. S. et al. Directed evolution of APEX2 for electron microscopy and proximity labeling. Nat. Methods 12, 51–54 (2015).
Branon, T. C. et al. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 36, 880–887 (2018).
Wales, P. et al. Calcium-mediated actin reset (CaAR) mediates acute cell adaptations. eLife 5, e19850 (2016).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Ridler, T. & Calvard, S. Picture thresholding using an iterative selection method. IEEE Trans. Syst. Man Cybern. 8, 630–632 (1978).
Lippincott-Schwartz, J., Snapp, E. L. & Phair, R. D. The development and enhancement of FRAP as a key tool for investigating protein dynamics. Biophys. J. 115, 1146–1155 (2018).
Borgese, N., Gazzoni, I., Barberi, M., Colombo, S. & Pedrazzini, E. Targeting of a tail-anchored protein to endoplasmic reticulum and mitochondrial outer membrane by independent but competing pathways. Mol. Biol. Cell 12, 2482–2496 (2001).
Omari, S. et al. Noncanonical autophagy at ER exit sites regulates procollagen turnover. Proc. Natl Acad. Sci. USA 115, E10099–E10108 (2018).
Subach, O. M., Cranfill, P. J., Davidson, M. W. & Verkhusha, V. V. An enhanced monomeric blue fluorescent protein with the high chemical stability of the chromophore. PLoS ONE 6, e28674 (2011).
Geiser, M., Cebe, R., Drewello, D. & Schmitz, R. Integration of PCR fragments at any specific site within cloning vectors without the use of restriction enzymes and DNA ligase. BioTechniques 31, 88–90 (2001).
Acknowledgements
We are grateful to S. Harada (Salk Institute for Biological Studies) for help with the cartoon diagram for Fig. 1. We thank C. Obara and J. Lippincott-Schwartz (Janelia Farms) for critical feedback and suggestions on the manuscript. This work was also greatly improved by feedback on our initial bioRxiv preprint received via Twitter. The Waitt Advanced Biophotonics Center is funded by the Waitt Foundation and Core Grant application National Cancer Institute (NCI) Cancer Center Support Grant (CCSG; CA014195). This work was supported by the Transgenic Core Facility of the Salk Institute with funding from the National Institutes of Health (NIH)–NCI CCSG (P30 014195). The laboratory of R.G. is funded by grants from the Human Frontier Science Program (HFSP; RGP0021/2016) and the Cluster of Excellence Centre for Integrative Biological Signaling Studies (CIBSS; EXC-2189). G.S.S. is supported by a NIH grant (no. R01 AR069876) and the Salk Institute Audrey Geisel Chair in Biomedical Science. The laboratory of O.A.Q. is supported by a National Institute of General Medical Sciences grant (NIGMS; R15 GM119077) and funding from the University of Richmond School of Arts & Sciences.
Author information
Authors and Affiliations
Contributions
C.R.S., T.Z., B.Z., O.A.Q., G.S.S., R.G. and U.M. planned the experimental design and data analysis; C.R.S., T.Z., A.S.M., L.R.A., M.W., J.W.F. and U.M. performed the experiments; C.R.S., T.Z., A.S.M., P.W. and J.W.F. performed data analysis and quantification; B.Z., T.C.S., Y.D., O.A.Q., G.S.S. and R.G. provided key reagents; U.M. supervised the study; and C.R.S., T.Z. and U.M. composed the figures and videos and wrote the manuscript with input from the rest of the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Rita Strack was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 AC-ER accumulates alongside mitochondria.
Left: five high magnification examples of AC-ER accumulating specifically alongside mitochondria are shown. Note that the mCherry-ER control probe does not specifically accumulate in these regions. The degree of correlation with the MitoTracker channel (Mander’s correlation coefficient) is shown below each example for both the AC-ER and mCherry control probes. Scale bar: 1 µm. Right: three low magnification examples are shown. Maximum intensity projections are shown. Scale bars: 5 µm. These results were reproducible across 21 independent experiments.
Extended Data Fig. 2 AC probes provide higher sensitivity and specificity for imaging sub-organellar F-actin accumulation.
a) Maximum projection of cells stained with phalloidin and MitoTracker do not show obvious sites of accumulation of actin on mitochondria. Scale bar: 10µm. These results were reproducible across 12 independent experiments. b) Maximum projection of cells expressing an ER marker counterstained with phalloidin do not show obvious accumulation of actin on any specific region of the ER. Scale bar: 10µm. These results were reproducible across 12 independent experiments. c) Expression of AC probes does not alter actin accumulation on mitochondria or the ER. Left graph: HeLa cells were either not transfected or transfected with AC-mito, then stained with MitoTracker and phalloidin. Pearson’s correlation coefficient between MitoTracker and phalloidin was determined for each condition. Right graph: HeLa cells were either transfected with mCherry-ER only or cotransfected with mCherry-ER and AC-ER, then stained with phalloidin. Pearson’s correlation coefficient between mCherry-ER and phalloidin was determined for each condition. N = 10 cells per condition. P-values determined by two-tailed Welch’s t-test. d) Quantification of the percentage of mitochondria or ER overlapped by AC probes, mCherry-tagged control probes, or F-actin probes. Our AC probes display more specific labeling compared to their corresponding controls or pan-actin probes. Quantification of overlap was carried out as displayed in E and described under Methods. Mitochondria overlap: AC-mito: n = 53 cells, AC-ER: n = 76 cells, mCherry-ER: n = 71 cells, F-actin: n = 26 cells. ER overlap: AC-mito: n = 20 cells, AC-ER: n = 73 cells, mCherry-mito: n = 71 cells, F-actin: n = 18 cells. P-values determined by two-tailed Welch’s t-test. e) An example of a HeLa cell expressing AC-mito and stained with MitoTracker and phalloidin. Scale bar: 10µm. The top row shows standard images of the cell. The center row shows the corresponding mitochondrial area in magenta and phalloidin or AC-mito area in green. Areas of overlap are shown in white and quantified. The lower left panel displays the area of overlap between mitochondria and phalloidin in cyan and the area of overlap between mitochondria and AC-mito in yellow. The area of overlap between the two is shown in white and quantified. The lower right panel summarizes the qunatification for this example. Green: percentage of mitochondria overlapped by phalloidin or AC-mito. Cyan: percentage of mitochondria overlapped by only phalloidin. This is the proportion of non-specific phalloidin overlap. Yellow: percentage of mitochondria overlapped by only AC-mito. This represents the proportion of actin that phalloidin lacks the sensitivity to detect. White: percentage of mitochondria overlapped by both phalloidin and AC-mito. These results were reproducible across 12 independent experiments. f) Further quantification of E across multiple cells either transfected with AC-mito and labelled with MitoTracker and phalloidin (left graph) or co-transfected with AC-ER and mCherry-ER and labelled with phalloidin (right graph). Averages and standard deviation are shown. N = 10 cells per condition. P-values determined by the two-tailed ratio paired t-test.
Extended Data Fig. 3 AC-mito expression does not alter endogenous Fis1 localization to mitochondria.
Maximum projection of cells expressing AC-mito and non-transfected cells show similar levels of endogenous anti-Fis1 immunofluorescence. Note that the anti-Fis1 antigen is the Fis1 N-terminus, which is not present in the AC-mito protein, which contains only the C-terminus of Fis1. Scale bar: 20 µm. These results were reproducible across 2 independent experiments.
Extended Data Fig. 4 Alternate membrane-targeting and actin-binding motifs yield similar results.
Switching the actin nanobody motif with LifeAct (Fis1-LifeAct-GFP) yields similarly increased accumulation compared to control constructs as quantified by the coefficient of variance. Switching the Fis1 mitochondrial outer membrane targeting sequence for Cytb5mito (Cytb5mito-AC-GFP) also yields similar results. Scale bar: 5 µm. The table displays the degree of accumulation as quantified by the average coefficient of variance (standard deviation/mean pixel intensity in the mitochondrial region) ± the standard deviation for each probe compared to control probes. N indicates the number of cells analyzed. P-values determined by two-tailed Welch’s t-test. These results were reproducible across 7 independent experiments.
Extended Data Fig. 5 Comparison of AC probe dynamics in “accumulated” vs. “diffuse” regions.
a) Example of a cell containing regions with accumulated vs. diffuse AC probe localization. Regions with AC-mito accumulation (“Accumulated”) and without AC-mito accumulation (“Diffuse”) are indicated. Panels in the lower row (“Clipped”) display thresholded AC-mito signal (displaying only the top 25% signal) while the top row displays the same region without thresholding. Scale bar: 5 µm. The mean pixel intensity in boxed regions was determined for AC-mito and the mCherry-mito control probe and the ratio was calculated (mean pixel intensity in accumulated region/mean pixel intensity in diffuse region). The results for the example shown are displayed below the panels and quantification across multiple cells (n= 12 cells) is displayed in the scatter plot. Each dot color corresponds to an individual region in which pixel intensity was measured. P-values determined via a two-tailed ratio paired t-test. These results were reproducible across 9 independent experiments. b) Example of AC-ER accumulation. Same as A, showing results for AC/mCherry-ER. c) AC-mito and mCherry-mito have similar mobility in diffuse regions. FRAP was performed on the indicated accumulated and diffuse regions. Fluorescence recovery over time for the example shown is displayed beneath the image. Quantification of the mobile fraction (top scatter plot) and t1/2 (bottom scatter plot) across multiple cells is also displayed. Accumulated regions: n = 10 cells, diffuse regions: n = 8 cells. P-values determined by two-tailed Welch’s t-test. These results were reproducible across 3 independent experiments.
Extended Data Fig. 6 Actin depolymerization destroys AC-mito and AC-ER accumulation.
a) U2OS cells stained with MitoTracker and expressing AC-mito and pan-AC (cytoplasmic AC-tagRFP) were imaged before and after treatment with 2 µM Latrunculin B (LatB) for 30 minutes. After LatB treatment, AC-mito displayed significantly reduced accumulation at specific regions on mitochondria. This loss of accumulation was observed in all cells imaged after treatment with LatB or Cytochalasin D (n = 6 cells). The magnified images in the bottom row show an example of LatB treatment causing actin aggregates which colocalize with AC-mito. Maximum intensity projections are shown. Scale bar: 5 µm. These results were reproducible across 5 independent experiments. b) HeLa cells co-expressing AC-mito and the mCherry-mito control probe before and after treatment with LatB including example insets showing loss of AC-mito accumulation following LatB treatment. The yellow line in the merged inset indicates the area used to generate the line scan. The coefficient of variance was calculated before and after LatB treatment in the boxed region as described under Methods. Scale bar: 5 µm. These results were reproducible across 5 independent experiments. c) HeLa cells co-expressing AC-ER and the mCherry-ER control probe before and after treatment with LatB. The white box marks an area where AC-ER accumulation is lost following LatB treatment. The coefficient of variance for mCherry/AC-ER was calculated before and after LatB treatment in the white boxed region. Yellow arrows label areas of mCherry/AC-ER aggregation that result from LatB treatment. Higher magnification versions of some of these regions (marked by the yellow boxes) are shown in the bottom row. Scale bar: 5 µm. These results were reproducible across 5 independent experiments. d) FRAP-based quantificaton of mobile fractions before and after LatB treatment. FRAP was performed on cells co-expressing the mCherry-mito control probe and AC-mito (left graph) or the mCherry-ER control probe and AC-ER (right graph) before and after treatment with LatB. The mobile fraction of each probe was calculated and is displayed as a ratio of the mobile fracton after LatB treatment/the mobile fraction before LatB treatment. The AC probe mobility is increased following LatB treatment while the mobility of the corresponding mCherry control probes is unchanged. N = 7 cells per condition. P-values determined by two-tailed ratio paired t-test. These results were reproducible across 3 independent experiments.
Extended Data Fig. 7 AC-ER dynamically co-accumulates with F-actin during actin wave cycling.
Actin waves were imaged in HeLa cells co-expressing AC-ER and the F-actin marker LifeAct. Boxes mark regions of LifeAct accumulation at different timepoints. Graphs displaying normalized pixel intensity over time in the boxed regions are displayed as insets. This figure represents chosen timepoints from Supplementary Videos 8 and 9. The actin wave dynamics are much more easily visualized in the movies. Scale bar: 5 µm. These results were reproducible across 4 independent experiments.
Extended Data Fig. 8 Impact of ionomycin treatment on cells expressing AC probes.
a) HeLa cells stained with MitoTracker and expressing AC-mito and pan-AC (cytoplasmic AC-tagRFP) were treated with 10 µM ionomycin for 10 minutes. Fragmented mitochondria surrounded by both pan-AC and AC-mito can be visualized. Scale bar: 5 µm. These results were reproducible across 5 independent experiments. b) HeLa cells expressing AC-mito or AC-ER and counterstained with MitoTracker were treated with 10 µM ionomycin and imaged for 10 minutes. The time after ionomycin addition when mitochondrial fragmentation became evident was recorded for each cell. Cells from the same field displaying no AC-mito or AC-ER signal were considered untransfected. AC-mito: n= 11 cells, AC-ER: n = 24 cells. P-values determined by two-tailed Welch’s t-test.
Extended Data Fig. 9 Mitochondria- and ER-associated actin accumulates during Drp1-mediated mitochondrial fission.
a) AC-ER accumulates prior to Drp1-mediated fission. AC-ER accumulation is evident prior to Drp1 recruitment and mitochondrial fission. Hollow arrows mark the location of mitochondrial fission. Yellow lines in the left column mark the area used to generate the line scans. Scale bar: 1 µm. These results were reproducible across 8 independent experiments. b) AC-mito accumulates prior to Drp1-mediated fission. Same as A with cells expressing AC-mito instead of AC-ER. c) Expression of AC probes does not alter mitochondrial membrane potential, mitochondrial fission rate, or ER-mitochondria contact sites. Top graphs: the pixel intensity of MitoTracker Deep Red (MTDR) was measured in cells that were not transfected vs. neighboring cells expressing AC-mito/ER and normalized as described under Methods. Left graph: non-transfected: n = 23 cells, AC-ER: n = 19 cells. Right graph: non-transfected: n = 14 cells, AC-mito: n = 9 cells. P-values determined by two-tailed Welch’s t-test. Bottom left graph: the number of fission events were counted in cells that were not transfected or in cells expressing AC-mito/ER. Non-transfected: n = 17 cells, AC-ER: n = 8 cells, AC-mito: n = 12 cells. P-values determined by two-tailed Welch’s t-test. Bottom right graph: HeLa cells were transfected with mCherry-ER alone “No AC Probe”, AC-ER+mCherry-ER “AC-ER”, AC-mito+mCherry-ER “AC-mito, or VAPB1-Halo+mCherry-ER “VAPB1” (positive control for changing ER-mitochondria contacts) and counterstained with MitoTracker. A region of the cell was selected as described under Methods and Pearson’s correlation coefficient between MitoTracker and mCherry-ER was determined. N = 10 cells per condition. P-values determined by two-tailed Welch’s t-test.
Extended Data Fig. 10 Mitochondria- and ER-associated actin accumulates during ER-mediated mitochondrial fission.
a) AC-ER specifically accumulates at fission sites prior to ER-mediated fission. AC-ER accumulation is evident during ER-mediated mitochondrial fission. Hollow arrows mark the location of mitochondrial fission. Yellow lines in the left column mark the area used to generate the line scans. Scale bar: 1 µm. Example images of the areas used to determine the degree of MitoTracker overlap and coefficient of variance for mCherry/AC-ER and the resulting values are also shown. These results were reproducible across 19 independent experiments. b) AC-mito accumulates prior to ER-mediated fission. Same as A with cells expressing AC-mito instead of AC-ER.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9 and Supplementary Table 1
Supplementary Video 1
Live-cell imaging of mitochondria-associated actin. A Hap1 cell expressing AC-mito counterstained with MitoTracker shows dynamic subdomains of actin enrichment on mitochondria.
Supplementary Video 2
Live-cell imaging of mitochondria-associated actin. A Hap1 cell expressing AC-mito counterstained with MitoTracker shows dynamic subdomains of actin enrichment on mitochondria.
Supplementary Video 3
Live-cell imaging of ER-associated actin. A Hap1 cell expressing AC-ER counterstained with MitoTracker shows dynamic subdomains of actin enrichment on the ER, in particular at ER–mitochondria contact sites.
Supplementary Video 4
Live-cell imaging of ER-associated actin. A Hap1 cell expressing AC-ER counterstained with MitoTracker shows dynamic subdomains of actin enrichment on the ER, in particular at ER–mitochondria contact sites.
Supplementary Video 5
FRAP of cell coexpressing AC-mito and mCherry-mito. FRAP of U2OS cells coexpressing AC-mito and mCherry-mito shows that AC-mito is highly mobile but less mobile than mCherry-mito.
Supplementary Video 6
FRAP of cell coexpressing AC-ER and mCherry-ER. FRAP of U2OS cells coexpressing AC-ER and mCherry-ER shows that AC-ER is highly mobile but less mobile than mCherry-ER.
Supplementary Video 7
Live imaging of LifeAct and AC-mito during actin wave cycling. Co-accumulation of LifeAct and AC-mito is observable in several regions of the cell during actin wave cycling. Scale bar: 5 µm. Graphs display the normalized average pixel intensity over time within the indicated boxed regions. This video corresponds to Fig. 2b.
Supplementary Video 8
Live imaging of LifeAct and AC-ER during actin wave cycling (single cell). Co-accumulation of the F-actin marker LifeAct and AC-ER is observable during actin wave cycling. Scale bar: 5 µm. Graphs display the normalized average pixel intensity over time within the indicated boxed regions. This video corresponds to Extended Data Fig. 7.
Supplementary Video 10
Live imaging of AC-mito and Halo-mito during mitochondrial fission. Live imaging of AC-mito, Halo-mito (Halo-Fis1) and mitochondria (BFP-mito) in HeLa cells reveals accumulation of mitochondria-associated actin before fission. Scale bar, 1 µm. This video corresponds to Fig. 2d.
Supplementary Video 11
Live imaging of AC-mito and mitochondrial fragmentation with ionomycin treatment. Live imaging of AC-mito in HeLa cells counterstained with MitoTracker treated with 10 µM ionomycin reveals mitochondrial fragmentation. Fragmentation occurs simultaneously across cells regardless of AC-mito expression. Scale bar: 20 µm.
Supplementary Video 12
Live imaging of AC-ER and mitochondrial fragmentation with ionomycin treatment. Live imaging of AC-ER in HeLa cells counterstained with MitoTracker treated with 10 µM ionomycin reveals mitochondrial fragmentation. Fragmentation occurs simultaneously across cells regardless of AC-ER expression. Scale bar, 20 µm.
Supplementary Video 13
Live imaging of AC-mito during mitochondrial fission. Live imaging of AC-mito in HeLa cells counterstained with MitoTracker reveals accumulation of mitochondria-associated actin before fission. Scale bar, 0.5 µm. This video corresponds to Fig. 3a.
Supplementary Video 14
Live imaging of AC-ER during mitochondrial fission. Live imaging of AC-ER in HeLa cells coexpressing BFP-mito reveals accumulation of ER-associated actin before fission. Scale bar, 1 µm. This montage corresponds to Fig. 3b.
Supplementary Video 15
Live imaging of AC-ER during Drp1-mediated mitochondrial fission. Live imaging of AC-ER and mCherry-Drp1 in HeLa cells counterstained with MitoTracker reveals accumulation of ER-associated actin before Drp1 accumulation and mitochondrial fission. Scale bar, 1 µm. This video corresponds to Extended Data Fig. 9a.
Supplementary Video 16
Live imaging of AC-mito during Drp1-mediated mitochondrial fission. Live imaging of AC-mito and mCherry-Drp1 in HeLa cells counterstained with MitoTracker reveals accumulation of mitochondria-associated actin before Drp1 accumulation and mitochondrial fission. Scale bar, 1 µm. This video corresponds to Extended Data Fig. 9b.
Supplementary Video 17
Live imaging of AC-mito and mCherry-mito during mitochondrial fission. Live imaging of AC-mito and mCherry-mito in HeLa cells counterstained with MitoTracker reveals accumulation of mitochondria-associated actin before mitochondrial fission. Scale bar, 1 µm. This video corresponds to Supplementary Fig. 5.
Supplementary Video 18
Live imaging of AC-ER during ER-mediated mitochondrial fission. Live imaging of AC-ER and mCherry-ER in HeLa cells counterstained with MitoTracker reveals accumulation of ER-associated actin before ER-mediated mitochondrial fission. Scale bar, 1 µm. This video corresponds to Extended Data Fig. 10a.
Supplementary Video 19
Live imaging of AC-mito during ER-mediated mitochondrial fission. Live imaging of AC-mito and mCherry-ER in HeLa cells counterstained with MitoTracker reveals accumulation of mitochondria-associated actin before ER-mediated mitochondrial fission. Scale bar, 1 µm. This video corresponds to Extended Data Fig. 10b.
Supplementary Video 20
Example of artifact resulting from AC-mito overexpression. Live imaging of AC-mito in a HeLa cell counterstained with MitoTracker. Mild mitochondrial clustering and a decrease in mitochondrial movement is evident in the perinuclear region coinciding with a high degree of AC-mito signal. Scale bar, 10 µm.
Supplementary Video 21
Live imaging of AC-mito and AC-ER during mitochondrial fission. Live imaging of AC-mito and AC-ER in HeLa cells counterstained with MitoTracker reveals accumulation of mitochondria-associated actin before accumulation of ER-associated actin. Scale bar, 1 µm. This video corresponds to Fig. 3d.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
About this article
Cite this article
Schiavon, C.R., Zhang, T., Zhao, B. et al. Actin chromobody imaging reveals sub-organellar actin dynamics. Nat Methods 17, 917–921 (2020). https://doi.org/10.1038/s41592-020-0926-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-020-0926-5
This article is cited by
-
VAP spatially stabilizes dendritic mitochondria to locally support synaptic plasticity
Nature Communications (2024)
-
Mitochondrial DNA replication stress triggers a pro-inflammatory endosomal pathway of nucleoid disposal
Nature Cell Biology (2024)
-
Actin in action
Nature Methods (2023)
-
Quantitative structured illumination microscopy via a physical model-based background filtering algorithm reveals actin dynamics
Nature Communications (2023)
-
Automated segmentation and tracking of mitochondria in live-cell time-lapse images
Nature Methods (2021)