Abstract
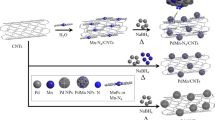
Electrochemical reduction of CO2 is a promising route for sustainable production of fuels. A grand challenge is developing low-cost and efficient electrocatalysts that can enable rapid conversion with high product selectivity. Here we design a series of nickel phthalocyanine molecules supported on carbon nanotubes as molecularly dispersed electrocatalysts (MDEs), achieving CO2 reduction performances that are superior to aggregated molecular catalysts in terms of stability, activity and selectivity. The optimized MDE with methoxy group functionalization solves the stability issue of the original nickel phthalocyanine catalyst and catalyses the conversion of CO2 to CO with >99.5% selectivity at high current densities of up to −300 mA cm−2 in a gas diffusion electrode device with stable operation at −150 mA cm−2 for 40 h. The well-defined active sites of MDEs also facilitate the in-depth mechanistic understandings from in situ/operando X-ray absorption spectroscopy and theoretical calculations on structural factors that affect electrocatalytic performance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors declare that the main data supporting the findings of this study are available within the article, its Supplementary Information and source data. Source data are provided with this paper.
References
Lewis, N. S. & Nocera, D. G. Powering the planet: chemical challenges in solar energy utilization. Proc. Natl Acad. Sci. USA 103, 15729–15735 (2006).
Davis, S. J., Caldeira, K. & Matthews, H. D. Future CO2 emissions and climate change from existing energy infrastructure. Science 329, 1330–1333 (2010).
Artero, V. & Fontecave, M. Solar fuels generation and molecular systems: is it homogeneous or heterogeneous catalysis? Chem. Soc. Rev. 42, 2338–2356 (2013).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Gao, S. et al. Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel. Nature 529, 68–71 (2016).
Dinh, C.-T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Fei, H. et al. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal. 1, 63–72 (2018).
Kortlever, R., Shen, J., Schouten, K. J. P., Calle-Vallejo, F. & Koper, M. T. M. Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide. J. Phys. Chem. Lett. 6, 4073–4082 (2015).
Zheng, T., Jiang, K. & Wang, H. Recent advances in electrochemical CO2-to-CO conversion on heterogeneous catalysts. Adv. Mater. 30, 1802066 (2018).
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Jiao, F. et al. Selective conversion of syngas to light olefins. Science 351, 1065–1068 (2016).
Chen, Y., Li, C. W. & Kanan, M. W. Aqueous CO2 reduction at very low overpotential on oxide-derived Au nanoparticles. J. Am. Chem. Soc. 134, 19969–19972 (2012).
Liu, M. et al. Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature 537, 382–386 (2016).
Dinh, C.-T., García de Arquer, F. P., Sinton, D. & Sargent, E. H. High rate, selective, and stable electroreduction of CO2 to CO in basic and neutral media. ACS Energy Lett. 3, 2835–2840 (2018).
Verma, S. et al. Insights into the low overpotential electroreduction of CO2 to CO on a supported gold catalyst in an alkaline flow electrolyzer. ACS Energy Lett. 3, 193–198 (2018).
Jhong, H.-R. M., Ma, S. & Kenis, P. J. A. Electrochemical conversion of CO2 to useful chemicals: current status, remaining challenges, and future opportunities. Curr. Opin. Chem. Eng. 2, 191–199 (2013).
Zhao, C. et al. Ionic exchange of metal–organic frameworks to access single nickel sites for efficient electroreduction of CO2. J. Am. Chem. Soc. 139, 8078–8081 (2017).
Ju, W. et al. Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO2. Nat. Commun. 8, 944 (2017).
Lieber, C. M. & Lewis, N. S. Catalytic reduction of carbon dioxide at carbon electrodes modified with cobalt phthalocyanine. J. Am. Chem. Soc. 106, 5033–5034 (1984).
Costentin, C., Drouet, S., Robert, M. & Savéant, J.-M. A local proton source enhances CO2 electroreduction to CO by a molecular Fe catalyst. Science 338, 90–94 (2012).
Lin, S. et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 349, 1208–1213 (2015).
Zhang, X. et al. Highly selective and active CO2 reduction electrocatalysts based on cobalt phthalocyanine/carbon nanotube hybrid structures. Nat. Commun. 8, 14675 (2017).
Ren, S. et al. Molecular electrocatalysts can mediate fast, selective CO2 reduction in a flow cell. Science 365, 367–369 (2019).
Zhu, M., Ye, R., Jin, K., Lazouski, N. & Manthiram, K. Elucidating the reactivity and mechanism of CO2 electroreduction at highly dispersed cobalt phthalocyanine. ACS Energy Lett. 3, 1381–1386 (2018).
Jiang, Z. et al. Revealing the hidden performance of metal phthalocyanines for CO2 reduction electrocatalysis by hybridization with carbon nanotubes. Nano Res. 12, 2330–2334 (2019).
Hu, X.-M., Rønne, M. H., Pedersen, S. U., Skrydstrup, T. & Daasbjerg, K. Enhanced catalytic activity of cobalt porphyrin in CO2 electroreduction upon immobilization on carbon materials. Angew. Chem. Int. Ed. 56, 6468–6472 (2017).
Zhu, M. et al. Covalently grafting cobalt porphyrin onto carbon nanotubes for efficient CO2 electroreduction. Angew. Chem. Int. Ed. 58, 6595–6599 (2019).
Choi, J. et al. Steric modification of a cobalt phthalocyanine/graphene catalyst to give enhanced and stable electrochemical CO2 reduction to CO. ACS Energy Lett. 4, 666–672 (2019).
Maurin, A. & Robert, M. Noncovalent immobilization of a molecular iron-based electrocatalyst on carbon electrodes for selective, efficient CO2-to-CO conversion in water. J. Am. Chem. Soc. 138, 2492–2495 (2016).
Wu, Y., Jiang, Z., Lu, X., Liang, Y. & Wang, H. Domino electroreduction of CO2 to methanol on a molecular catalyst. Nature 575, 639–642 (2019).
Li, X. et al. Exclusive Ni–N4 sites realize near-unity CO selectivity for electrochemical CO2 reduction. J. Am. Chem. Soc. 139, 14889–14892 (2017).
Yang, H. B. et al. Atomically dispersed Ni(i) as the active site for electrochemical CO2 reduction. Nat. Energy 3, 140–147 (2018).
Yan, C. et al. Coordinatively unsaturated nickel–nitrogen sites towards selective and high-rate CO2 electroreduction. Energy Environ. Sci. 11, 1204–1210 (2018).
Jiang, K. et al. Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction. Energy Environ. Sci. 11, 893–903 (2018).
Sorokin, A. B. Phthalocyanine metal complexes in catalysis. Chem. Rev. 113, 8152–8191 (2013).
Zhang, Z. et al. Reaction mechanisms of well-defined metal–N4 sites in electrocatalytic CO2 reduction. Angew. Chem. Int. Ed. 57, 16339–16342 (2018).
Clark, E. L. et al. Standards and protocols for data acquisition and reporting for studies of the electrochemical reduction of carbon dioxide. ACS Catal. 8, 6560–6570 (2018).
Weekes, D. M., Salvatore, D. A., Reyes, A., Huang, A. & Berlinguette, C. P. Electrolytic CO2 reduction in a flow cell. Acc. Chem. Res. 51, 910–918 (2018).
Higgins, D., Hahn, C., Xiang, C., Jaramillo, T. F. & Weber, A. Z. Gas-diffusion electrodes for carbon dioxide reduction: a new paradigm. ACS Energy Lett. 4, 317–324 (2019).
Yin, Z. et al. An alkaline polymer electrolyte CO2 electrolyzer operated with pure water. Energy Environ. Sci. 12, 2455–2462 (2019).
Möller, T. et al. Efficient CO2 to CO electrolysis on solid Ni–N–C catalysts at industrial current densities. Energy Environ. Sci. 12, 640–647 (2019).
Kutz, R. B. et al. Sustainion imidazolium-functionalized polymers for carbon dioxide electrolysis. Energy Technol. 5, 929–936 (2017).
Wang, M. et al. CO2 electrochemical catalytic reduction with a highly active cobalt phthalocyanine. Nat. Commun. 10, 3602 (2019).
Wu, Y. et al. Electroreduction of CO2 catalyzed by a heterogenized Zn–porphyrin complex with a redox-innocent metal center. ACS Cent. Sci. 3, 847–852 (2017).
Weng, Z. et al. Active sites of copper-complex catalytic materials for electrochemical carbon dioxide reduction. Nat. Commun. 9, 415 (2018).
Rollmann, L. D. & Iwamoto, R. T. Electrochemistry, electron paramagnetic resonance, and visible spectra of cobalt, nickel, copper, and metal-free phthalocyanines in dimethyl sulfoxide. J. Am. Chem. Soc. 90, 1455–1463 (1968).
Chen, L. X. et al. Capturing a photoexcited molecular structure through time-domain X-ray absorption fine structure. Science 292, 262–264 (2001).
Yamamoto, T. Assignment of pre-edge peaks in K-edge X-ray absorption spectra of 3d transition metal compounds: electric dipole or quadrupole? X-ray Spectrom. 37, 572–584 (2008).
Froehlich, J. D. & Kubiak, C. P. The homogeneous reduction of CO2 by [Ni(cyclam)]+: increased catalytic rates with the addition of a CO scavenger. J. Am. Chem. Soc. 137, 3565–3573 (2015).
Costentin, C. & Savéant, J.-M. Towards an intelligent design of molecular electrocatalysts. Nat. Rev. Chem. 1, 0087 (2017).
Kim, S.-J., Matsumoto, M. & Shigehara, K. Synthesis and electrical properties of poly(μ-1,4-diisocyanobenzene) octacyanophthalocyaninatoiron(ii). Synth. Met. 107, 27–33 (1999).
Yslas, E. I., Rivarola, V. & Durantini, E. N. Synthesis and photodynamic activity of zinc(ii) phthalocyanine derivatives bearing methoxy and trifluoromethylbenzyloxy substituents in homogeneous and biological media. Bioorg. Med. Chem. 13, 39–46 (2005).
Kim, S.-J., Matsumoto, M. & Shigehara, K. Synthesis and electrical properties of one-dimensional octacyanometallophthalocyanine (M≡Fe, Co) polymers. J. Porphyr. Phthalocyanines 04, 136–144 (2000).
Ma, M. et al. Insights into the carbon balance for CO2 electroreduction on Cu using gas diffusion electrode reactor designs. Energy Environ. Sci. 13, 977–985 (2020).
Wang, M., Árnadóttir, L., Xu, Z. J. & Feng, Z. In situ X-ray absorption spectroscopy studies of nanoscale electrocatalysts. Nano-Micro Lett. 11, 47 (2019).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Frisch, M. J. et al. Gaussian 09 (Gaussian, 2009).
Adamo, C. & Barone, V. Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Steinmetz, M. & Grimme, S. Benchmark study of the performance of density functional theory for bond activations with (Ni,Pd)-based transition-metal catalysts. ChemistryOpen 2, 115–124 (2013).
Andrae, D., Häußermann, U., Dolg, M., Stoll, H. & Preuß, H. Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 77, 123–141 (1990).
Hariharan, P. C. & Pople, J. A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 28, 213–222 (1973).
Gordon, M. S. The isomers of silacyclopropane. Chem. Phys. Lett. 76, 163–168 (1980).
Binning, R. & Curtiss, L. Compact contracted basis sets for third-row atoms: Ga–Kr. J. Comput. Chem. 11, 1206–1216 (1990).
Hirshfeld, F. L. Bonded-atom fragments for describing molecular charge densities. Theor. Chim. Acta 44, 129–138 (1977).
Lu, T. & Chen, F. Calculation of molecular orbital composition. Acta Chim. Sinica 69, 2393–2406 (2011).
Mayer, I. Charge, bond order and valence in the AB initio SCF theory. Chem. Phys. Lett. 97, 270–274 (1983).
Lu, T. & Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Buck, D. & Collins, A. Persistence of Vision Raytracer 3.6 (Persistence of Vision, 2004).
Chan, K. & Nørskov, J. K. Electrochemical barriers made simple. J. Phys. Chem. Lett. 6, 2663–2668 (2015).
Peterson, A. A., Abild-Pedersen, F., Studt, F., Rossmeisl, J. & Nørskov, J. K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 3, 1311–1315 (2010).
Acknowledgements
Y.L. acknowledges financial support from Shenzhen fundamental research funding (grant no. JCYJ20160608140827794) and Guangdong-Hong Kong-Macao Joint Laboratory for Photonic-Thermal-Electrical Energy Materials and Devices (grant no. 2019B121205001). H.W. acknowledges funding support from the US National Science Foundation (grant no. CHE-1651717). Z.F. thanks the start-up support from Oregon State University. Y.-G.W. acknowledges financial supports from Guangdong Provincial Key Laboratory of Catalysis (grant no. 2020B121201002). J.L. is supported by National Natural Science Foundation of China (grant nos. 21590792, 91426302 and 21433005). The computational resource is supported from the Center for Computational Science and Engineering (SUSTech) and Tsinghua National Laboratory for Information Science and Technology. TEM, MS and ICP data were obtained using equipment maintained by SUSTech Core Research Facilities. We acknowledge the technical support from R. Rosenberg at 4-ID, Advanced Photon Source (APS) of Argonne National Laboratory (ANL). XAS measurements were performed at 9-BM and DND-CAT 5-BM. The use of APS of ANL is supported by Department of Energy under contract no. DE-AC02-06CH11357. DND-CAT is supported through E. I. duPont de Nemours and Company, Northwestern University and The Dow Chemical Company.
Author information
Authors and Affiliations
Contributions
Y.L. and X.Z. conceived the project and designed the experiments. X.Z., Y.W., W.P. and Z.J. carried out the synthesis, material characterizations and electrochemical measurements. M.G. carried out the STEM characterizations. M.W., G.E.S., H.Z., M.L., Q.M. and Z.F. carried out the XAS characterizations. Z.Z., Y.-G.W. and J.L. performed the DFT calculations. Y.L., X.Z., H.W. and H.D. analysed the data. Y.L., X.Z. and H.D. prepared the manuscript with input from all the authors. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–31, Tables 1–4 and refs. 1–15.
Source data
Source Data Fig. 1
Statistical source data for Fig. 1.
Source Data Fig. 2
Statistical source data for Fig. 2.
Rights and permissions
About this article
Cite this article
Zhang, X., Wang, Y., Gu, M. et al. Molecular engineering of dispersed nickel phthalocyanines on carbon nanotubes for selective CO2 reduction. Nat Energy 5, 684–692 (2020). https://doi.org/10.1038/s41560-020-0667-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-020-0667-9
This article is cited by
-
Pulsed co-electrolysis of carbon dioxide and nitrate for sustainable urea synthesis
Nature Sustainability (2024)
-
Oxygen-tolerant CO2 electroreduction over covalent organic frameworks via photoswitching control oxygen passivation strategy
Nature Communications (2024)
-
Guanine-derived F, N co-doped carbon-shell encapsulated iron carbide nanoparticles for enhanced CO2 electroreduction activity
Nano Research (2024)
-
A general and facile calcination method to synthesize single-site catalysts for highly efficient electrochemical CO2 reduction
Nano Research (2024)
-
Tuning electronic properties of cobalt phthalocyanines for oxygen reduction and evolution reactions
Science China Chemistry (2024)