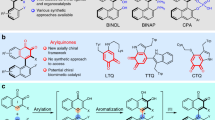
Abstract
Axially chiral biaryls are common structural motifs in functional materials, bioactive natural products, pharmaceuticals and chiral catalysts/ligands. As such, efficient preparation of these privileged scaffolds is an important endeavour in organic chemistry. Herein we report a general and modular platform technology for the construction of axial chirality via palladium/chiral norbornene cooperative catalysis. It is a three-component cascade process that involves widely available aryl iodides, 2,6-substituted aryl bromides and olefins (or alkynes, boronic acids and so on) as the reactants. A wide variety of substrates bearing an assortment of functional groups (88 examples) are compatible with this method. Other features include a distinct stereoinduction model, excellent enantioselectivities, step economy and scalability. This method is also amenable for the synthesis of chiral fluorenols through axial-to-central chirality transfer in high stereochemical fidelity. We anticipate that this work will have broad synthetic utilities in chiral ligands and catalyst-design for asymmetric catalysis.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data relating to the materials, optimization studies, experimental procedures and characterization of the new compounds are available in the Supplementary Information. Crystallographic data for 4g, 4da and 5j are available free of charge from the Cambridge Crystallographic Database Centre (CCDC) under reference numbers 1946138, 1946094 and 1946139, respectively. All other data are available from the authors on reasonable request.
References
Kumarasamy, E., Raghunathan, R., Sibi, M. P. & Sivaguru, J. Nonbiaryl and heterobiaryl atropisomers: molecular templates with promise for atropselective chemical transformations. Chem. Rev. 115, 11239–11300 (2015).
Li, Q. et al. Reversible photoswitchable axially chiral dopants with high helical twisting power. J. Am. Chem. Soc. 129, 12908–12909 (2007).
Smyth, J. E., Butler, N. M. & Keller, P. A. A twist of nature–the significance of atropisomers in biological systems. Nat. Prod. Rep. 32, 1562–1583 (2015).
Clayden, J., Moran, W. J., Edwards, P. J. & LaPlante, S. R. The challenge of atropisomerism in drug discovery. Angew. Chem. Int. Ed. 48, 6398–6401 (2009).
Noyori, R. & Takaya, H. BINAP: an efficient chiral element for asymmetric catalysis. Acc. Chem. Res. 23, 345–350 (1990).
Chen, Y., Yekta, S. & Yudin, A. K. Modified BINOL ligands in asymmetric catalysis. Chem. Rev. 103, 3155–3211 (2003).
Parmar, D., Sugiono, E., Raja, S. & Rueping, M. Complete field guide to asymmetric BINOL-phosphate derived Brønsted acid and metal catalysis: history and classification by mode of activation; Brønsted acidity, hydrogen bonding, ion pairing, and metal phosphates. Chem. Rev. 114, 9047–9153 (2014).
Shirakawa, S., Wu, X. & Maruoka, K. Kinetic resolution of axially chiral 2-amino-1,1′-biaryls by phase-transfer-catalyzed N-allylation. Angew. Chem. Int. Ed. 52, 4312–4348 (2013).
Chen, J. et al. Carbonyl catalysis enables a biomimetic asymmetric Mannich reaction. Science 360, 1438–1442 (2018).
Wencel-Delord, J., Panossian, A., Leroux, F. R. & Colobert, F. Recent advances and new concepts for the synthesis of axially stereoenriched biaryls. Chem. Soc. Rev. 44, 3418–3430 (2015).
Loxq, P., Manoury, E., Poli, R., Deydier, E. & Labande, A. Synthesis of axially chiral biaryl compounds by asymmetric catalytic reactions with transition metals. Coord. Chem. Rev. 308, 131–190 (2016).
Yang, H., Yang, X. & Tang, W. Transition-metal catalyzed asymmetric carbon–carbon cross-coupling with chiral ligands. Tetrahedron 72, 6143–6174 (2016).
Hazra, C. K., Dherbassy, Q., Wencel-Delord, J. & Colobert, F. Synthesis of axially chiral biaryls through sulfoxide-directed asymmetric mild C–H activation and dynamic kinetic resolution. Angew. Chem. Int. Ed. 53, 13871–13875 (2014).
Gustafson, J. L., Lim, D. & Miller, S. J. Dynamic kinetic resolution of biaryl atropisomers via peptide-catalyzed asymmetric bromination. Science 328, 1251–1255 (2010).
Ma, G. & Sibi, M. P. Catalytic kinetic resolution of biaryl compounds. Chem. Eur. J. 21, 11644–11657 (2015).
Liao, G., Zhou, T., Yao, Q.-J. & Shi, B.-F. Recent advances in the synthesis of axially chiral biaryls via transition metal-catalysed asymmetric C–H functionalization. Chem. Commun. 55, 8514–8523 (2019).
Giri, R., Shi, B.-F., Engle, K. M., Maugel, N. & Yu, J.-Q. Transition metal-catalyzed C–H activation reactions: diastereoselectivity and enantioselectivity. Chem. Soc. Rev. 38, 3242–3272 (2009).
Hayashi, T., Hayashizaki, K., Kiyoi, T. & Ito, Y. Asymmetric synthesis catalyzed by chiral ferrocenylphosphine-transition-metal complexes. 6 Practical asymmetric synthesis of 1,1′-binaphthyls via asymmetric cross-coupling with a chiral [(alkoxyalkyl)ferrocenyl]monophosphine/nickel catalyst. J. Am. Chem. Soc. 110, 8153–8156 (1988).
Shen, D., Xu, Y. & Shi, S.-L. A bulky chiral N‑heterocyclic carbene palladium catalyst enables highly enantioselective Suzuki–Miyaura cross-coupling reactions for the synthesis of biaryl atropisomers. J. Am. Chem. Soc. 141, 14938–14945 (2019).
Qi, L.-W., Li, S., Xiang, S.-H., Wang, J. & Tan, B. Asymmetric construction of atropisomeric biaryls via a redox neutral cross-coupling strategy. Nat. Catal. 2, 314–323 (2019).
Link, A. & Sparr, C. Stereoselective arene formation. Chem. Soc. Rev. 47, 3804–3815 (2018).
Zhao, K. et al. Enhanced reactivity by torsional strain of cyclic diaryliodonium in Cu-catalyzed enantioselective ring-opening reaction. Chem 4, 599–612 (2018).
Wang, Y.-B. & Tan, B. Construction of axially chiral compounds via asymmetric organocatalysis. Acc. Chem. Res. 51, 534–547 (2018).
Yamaguchi, K., Yamaguchi, J., Studer, A. & Itami, K. Hindered biaryls by C–H coupling: bisoxazoline-Pd catalysis leading to enantioselective C–H coupling. Chem. Sci. 3, 2165–2169 (2012).
Jia, Z.-J. et al. General enantioselective C–H activation with efficiently tunable cyclopentadienyl ligands. Angew. Chem. Int. Ed. 56, 2429–2434 (2017).
Jang, Y.-S., Woźniak, Ł., Pedroni, J. & Cramer, N. Access to P‐ and axially chiral biaryl phosphine oxides by enantioselective Cpx IrIII atalyzed C–H arylations. Angew. Chem. Int. Ed. 57, 12901–12905 (2018).
Catellani, M., Frignani, F. & Rangoni, A. A complex catalytic cycle leading to a regioselective synthesis of o,o′‐disubstituted vinylarenes. Angew. Chem. Int. Ed. 36, 119–122 (1997).
Kim, D.-S., Park, W.-J. & Jun, C.-H. Metal–organic cooperative catalysis in C–H and C–C bond activation. Chem. Rev. 117, 8977–9015 (2017).
Catellani, M., Motti, E. & Ca’, N. D. Catalytic sequential reactions involving palladacycle-directed aryl coupling steps. Acc. Chem. Res. 41, 1512–1522 (2008).
Ding, L., Sui, X. & Gu, Z. Enantioselective synthesis of biaryl atropisomers via Pd/norbornene-catalyzed three-component cross-couplings. ACS Catal. 8, 5630–5635 (2018).
Zhao, Y.-B. et al. Exploiting the divergent reactivity of aryl–palladium intermediates for the rapid assembly of fluorene and phenanthrene derivatives. Angew. Chem. Int. Ed. 48, 1849–1852 (2009).
Yang, L. et al. Palladium-catalyzed dynamic kinetic asymmetric transformation of racemic biaryls: axial-to-central chirality transfer. J. Am. Chem. Soc. 137, 4876–4879 (2015).
Shi, H., Herron, A. N., Shao, Y., Shao, Q. & Yu, J.-Q. Enantioselective remote meta-C–H arylation and alkylation via a chiral transient mediator. Nature 558, 581–585 (2018).
Li, R., Liu, F. & Dong, G. Palladium-catalyzed asymmetric annulation between aryl iodides and racemic epoxides using a chiral norbornene cocatalyst. Org. Chem. Front. 5, 3108–3112 (2018).
Cao, Z. et al. Pd-catalyzed asymmetric allylic alkylation of indoles and pyrroles by chiral alkene-phosphine ligands. Org. Lett. 13, 2164–2167 (2011).
Shintani, R., Duan, W.-L., Okamoto, K. & Hayashi, T. Palladium/chiral phosphine–olefin complexes: X-ray crystallographic analysis and the use in catalytic asymmetric allylic alkylation. Tetrahedron Asymmetry 16, 3400–3405 (2005).
Liu, Z. & Du, H. Development of chiral terminal-alkene-phosphine hybrid ligands for palladium-catalyzed asymmetric allylic substitutions. Org. Lett. 12, 3054–3057 (2010).
Lam, F. L. et al. Palladium–(S,pR)-ferroNPS-catalyzed asymmetric allylic etherification: electronic effect of nonconjugated substituents on benzylic alcohols on enantioselectivity. Angew. Chem. Int. Ed. 47, 1280–1283 (2008).
Wen, W. et al. Chiral aldehyde catalysis for the catalytic asymmetric activation of glycine esters. J. Am. Chem. Soc. 140, 9774–9780 (2018).
Witzig, R. M., Fäseke, V. C., Häussinger, D. & Sparr, C. Atroposelective synthesis of tetra-ortho-substituted biaryls by catalyst-controlled non-canonical polyketide cyclizations. Nat. Catal. 2, 925–930 (2019).
Acknowledgements
We are grateful to the National Natural Science Foundation of China (grant nos. 21871213 and 21801193), the China Postdoctoral Science Foundation (grant nos. 2016M602339 and 2018M642894) and the start-up funding from WHU for financial support. We thank H. Cong and W. Yan (Wuhan University) for X-ray crystallographic analysis assistance. We gratefully acknowledge P. Baran (TSRI), D. Ma and W. Tang (SIOC), H. Xu (Georgia State University), C. Wang and W.-B. Liu (Wuhan University), S. Yu (Nanjing University) and W. Xie (Northwest A&F University) for helpful discussions, H. Xu (Georgia State University) and M. Yan (Fish & Richardson) for help with preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Q.Z. and Z.-S.L. conceived the idea. Q.Z. guided the project and wrote the manuscript. Z.-S.L., Y.H., Q.G., Y.M., H.T. and Y.S. performed the experiments and analysed the data. Z.-S.L. and H.-G.C. participated in the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–11, Tables 1–17 and references.
Supplementary Data 1
Crystallographic Data of compound 4da.
Supplementary Data 2
Crystallographic Data of compound 4g.
Supplementary Data 3
Crystallographic Data of compound 5j.
Rights and permissions
About this article
Cite this article
Liu, ZS., Hua, Y., Gao, Q. et al. Construction of axial chirality via palladium/chiral norbornene cooperative catalysis. Nat Catal 3, 727–733 (2020). https://doi.org/10.1038/s41929-020-0494-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-0494-1
This article is cited by
-
Synthesis of axially chiral alkenylboronates through combined copper- and palladium-catalysed atroposelective arylboration of alkynes
Nature Synthesis (2023)
-
Regulation of biological processes by intrinsically chiral engineered materials
Nature Reviews Materials (2023)
-
Recent advances in the construction of axially chiral arylpyrroles
Science China Chemistry (2023)
-
Manipulations of phenylnorbornyl palladium species for multicomponent construction of a bridged polycyclic privileged scaffold
Communications Chemistry (2022)
-
Atroposelective isoquinolinone synthesis through cobalt-catalysed C–H activation and annulation
Nature Synthesis (2022)