Abstract
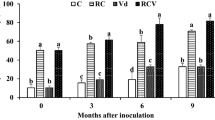
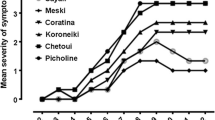
The present work aimed to investigate the effect of an autochthonous mycorrhizal consortium in enhancing olive tree tolerance against Verticillium dahliae. The bio-protective effect of this consortium “Rhizolive” on olive “Picholine Marocaine cultivar” infected with V. dahliae was assessed through monitoring disease symptoms development and through measuring the activities of enzymes involved in basal defense and secondary metabolism. The results revealed that “Rhizolive consortium” significantly (p < 0.05) reduced disease severity and incidence by 31% and 29%, respectively. The defoliation rate was also reduced by 35% in mycorrhizal-infected olive plants. “Rhizolive consortium” increased the lignin deposition and stimulated the phenylpropanoid pathway by increasing the phenylalanine ammonia-lyase (PAL) activity and accumulation of phenolic compounds in both roots and stems of plants infected with V. dahliae. The reduction in disease severity was accompanied by increased levels of lignin concentration, PAL activity and polyphenol content, particularly in the stems of olive plants. Our results clearly show that “Rhizolive consortium” inoculum protects olive tree from V. dahliae attacks and could thus constitute a biological approach contributing to the suppression of this telluric vascular pathogen.






Similar content being viewed by others
References
Abdel-Fattah GM, El-Haddad SA, Hafez EE, Rashad YM (2011) Induction of defense responses in common bean plants by arbuscular mycorrhizal fungi. Microbiol Res 166:268–281. https://doi.org/10.1016/j.micres.2010.04.004
Al-Askar AA, Rashad YM (2010) Arbuscular mycorrhizal fungi: a biocontrol agent against common bean Fusarium root rot disease. Plant Pathol J 9:31–38. https://doi.org/10.3923/ppj.2010.31.38
Anonyme (2020) Filiere vegetale. Chiffres clés oleiculture. https://www.fellah-trade.com/fr/filiere-vegetale/chiffres-cles-oleiculture. Consulté 08/02/2020
Antunes PM, Koch AM, Morton JB, Rillig MC, Klironomos JN (2011) Evidence for functional divergence in arbuscular mycorrhizal fungi from contrasting climatic origins. New Phytol 189:507–514
Azcón-Aguilar C, Jaizme-Vega MC, Calvet C (2002) The contribution of arbuscular mycorrhizal fungi to the control of soil-borne plant pathogens. In: Mycorrhizal technology in agriculture, pp 187–197
Báidez AG, Gómez P, Del Río JA, Ortuño A (2007) Dysfunctionality of the xylem in Olea europaea L. plants associated with the infection process by Verticillium dahliae Kleb. role of phenolic compounds in plant defense mechanism. J Agric Food Chem 55:3373–3377. https://doi.org/10.1021/jf063166d
Baslam M, Garmendia I, Goicoechea N (2011) Arbuscular mycorrhizal fungi (AMF) improved growth and nutritional quality of greenhouse-grown lettuce. Agric Food Chem 59:5504–5515
Ben Salah I, Aghrouss S, Douira A, Aissam S, El Alaoui-Talibi Z, Filali-Maltouf A, El Modafar C (2018) Seaweed polysaccharides as bio-elicitors of natural defenses in olive trees against verticillium wilt of olive. J Plant Interact 13:248–255. https://doi.org/10.1080/17429145.2018.1471528
Bibi N, Ahmed IM, Fan K, Dawood M, Li F, Yuan S, Wang X (2017) Role of brassinosteroids in alleviating toxin-induced stress of Verticillium dahliae on cotton callus growth. Environ Sci Pollut Res 24:12281–12292. https://doi.org/10.1007/s11356-017-8738-6
Boutaj H, Meddich A, Wahbi S, Moukhli A, El Alaoui-Talibi Z, Douira A, Filali-Maltouf A, El Modafar C (2019) Effect of Arbuscular Mycorrhizal Fungi on Verticillium wilt development of olive trees caused by Verticillium dahliae. Res J Biotechnol 14:79–88
Boutaj H, Chakhchar A, Meddich A, Wahbi S, El Alaoui-talibi Z, Douira A, Filali-maltouf A, El Modafar C (2020) Bioprotection of olive tree from Verticillium wilt by autochthonous endomycorrhizal fungi. J Plant Dis Prot 1:1–11. https://doi.org/10.1007/s41348-020-00323-z
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Bridi R, Troncoso MJ, Folch-Cano C, Fuentes J, Speisky H, López-Alarcón C (2014) A polyvinylpolypyrrolidone (PVPP)-assisted folin–ciocalteu assay to assess total phenol content of commercial beverages. Food Anal Methods 7:2075–2083. https://doi.org/10.1007/s12161-014-9856-0
Bubici G, Cirulli M (2012) Control of Verticillium wilt of olive by resistant rootstocks. Plant Soil 352:363–376. https://doi.org/10.1007/s11104-011-1002-9
Campbell CL, Madden LV (1990) Introduction to plant disease epidemiology. Wiley, New York, p 532
Cheffi M, Bouket AC, Alenezi FN, Luptakova L, Belka M, Vallat A, Rateb ME, Tounsi S, Triki MA (2019) Olea europaea L. Root Endophyte Bacillus velezensis OEE1 counteracts oomycete and fungal harmful pathogens and harbours a large repertoire of secreted and volatile metabolites and beneficial functional genes. Microorganisms 7:314. https://doi.org/10.3390/microorganisms7090314
Chérif M, Arfaoui A, Rhaiem A (2007) Phenolic compounds and their role in bio-control and resistance of chickpea to fungal pathogenic attacks. Tunis J Plant Prot 2:7–21
Chliyeh M, Touhami AO, Filali-maltouf A, El Modafar C, Moukhli A, Oukabli A, Benkirane R, Douira A (2014) Effect of a composite endomycorrhizal inoculum on the growth of olive trees under nurseries conditions in Morocco. Int J Pure Appl Biosci 2:1–14
Cui Y, Bell AA, Joost O, Magill C (2000) Expression of potential defense response genes in cotton. Physiol Mol Plant Pathol 56:25–31. https://doi.org/10.1006/pmpp.1999.0245
Dehne HW, Schönbeck F (1979) Untersuchungen zum Einfluß der endotrophen Mycorrhiza auf Pflanzenkrankheiten. J Phytopathol 95:210–216. https://doi.org/10.1111/j.1439-0434.1979.tb01595.x
Eke P, Chatue Chatue G, Wakam LN, Kouipou RMT, Fokou PVT, Boyom FF (2016) Mycorrhiza consortia suppress the fusarium root rot (Fusarium solani f. sp. Phaseoli) in common bean (Phaseolus vulgaris L.). Biol Control 103:240–250. https://doi.org/10.1016/j.biocontrol.2016.10.001
El Aabidine AZ, Baissac Y, Moukhli A, Jay-allemand C, Khadari B, El MC (2010) Resistance of olive tree to Spilocaea oleagina is mediated by the synthesis of phenolic compounds. Int J Agric Biol 12:61–67
El Modafar C (2010) Mechanisms of date palm resistance to Bayoud disease: current state of knowledge and research prospects. Physiol Mol Plant Pathol 74:287–294. https://doi.org/10.1016/j.pmpp.2010.06.008
El Modafar C, El Boustani E (2001) Cell wall-bound phenolic acid and lignin contents in date palm as related to its resistance to Fusarium oxysporum. Biol Plant 44:125–130. https://doi.org/10.1023/A:1017942927058
El Modafar C, El Boustani E (2005) The role of phenolics in plant defence mechanisms. In: Regnault-Roger C, Philogène BJR, Vincent C (eds) Biopesticides plant origin. Springer, London, pp 157–172
El Modafar C, Tantaoui A, El Boustani E (2000) Changes in cell wall-bound phenolic compounds and lignin in roots of date palm cultivars differing in susceptibility to Fusarium oxysporum f. sp. albedinis. J Phytopathol 148:405–411. https://doi.org/10.1046/j.1439-0434.2000.00512.x
El Said SH, Hegazi AA, Allatif AMA (2012) Resistance of some olive cultivars to Verticillium wilt. J Appl Sci Res 8:2758–2765
Espinosa F, Garrido I, Ortega A, Casimiro I, Álvarez-Tinaut MC (2014) Redox activities and ROS, NO and phenylpropanoids production by axenically cultured intact olive seedling roots after interaction with a mycorrhizal or a pathogenic fungus. PLoS ONE 9:e100132. https://doi.org/10.1371/journal.pone.0100132
Eynck C, Koopmann B, Karlovsky P, Von Tiedemann A (2009) Internal resistance in winter oilseed rape inhibits systemic spread of the vascular pathogen Verticillium longisporum. Phytopathology 99:802–811. https://doi.org/10.1094/PHYTO-99-7-0802
Garcia-Garrido JM (2009) Arbuscular mycorrhizae as defense against pathogens. In: White JF, Torres MS (eds) Defensive mutualism in microbial symbiosis. CRC Press, Boca Raton, pp 183–198
Garcia-Ruiz GM, Trapero C, Del Rio C, Lopez-Escudero FJ (2014) Evaluation of resistance of Spanish olive cultivars to Verticillium dahliae in inoculations conducted in greenhouse. Phytoparasitica 2:205–212. https://doi.org/10.1007/s12600-013-0353-6
Garmendia I, Goicoechea N, Aguirreolea J (2004) Plant phenology influences the effect of mycorrhizal fungi on the development of Verticillium-induced wilt in pepper. Eur J Plant Pathol 110:227–238
Garmendia I, Aguirreolea J, Goicoechea N (2006) Defence-related enzymes in pepper roots during interactions with arbuscular mycorrhizal fungi and/or Verticillium dahliae. Biocontrol 51:293. https://doi.org/10.1007/s10526-005-4238-6
Gayoso C, Pomar F, Novo-Uzal E, Merino F, Martínez de Ilárduya Ó (2010) The Ve-mediated resistance response of the tomato to Verticillium dahliae involves H2O2, peroxidase and lignins and drives PAL gene expression. BMC Plant Biol 10:232. https://doi.org/10.1186/1471-2229-10-232
Gharbi Y, Barkallah M, Bouazizi E, Gdoura R, Triki MA (2017) Differential biochemical and physiological responses of two olive cultivars differing by their susceptibility to the hemibiotrophic pathogen Verticillium dahliae. Physiol Mol Plant Pathol 97:30–39. https://doi.org/10.1016/j.pmpp.2016.12.001
Goicoechea N (2006) Verticillium-induced wilt in pepper: physiological disorders and perspectives for controlling the disease. Plant Pathol J 5:258–265. https://doi.org/10.3923/ppj.2006.258.265
Harrier LA, Watson CA (2004) The potential role of arbuscular mycorrhizal (AM) fungi in the bioprotection of plants against soil-borne pathogens in organic and/or other sustainable farming systems. Pest Manag Sci 60:149–157. https://doi.org/10.1002/ps.820
Hibilik N, Selmaoui K, Msairi S, Artib M, Chliyeh M, Ouazzani TA, Benkirane R, Douira A (2019) Behavior of a composite endomycorrhizal inoculum in the leek. Plant Arch 19:2029–2044
Jeong H-S, Lee J, Eom A-H (2006) Effects of interspecific interactions of arbuscular mycorrhizal fungi on growth of soybean and corn. Mycobiology 34:34–37. https://doi.org/10.4489/myco.2006.34.1.034
Jiménez-Moreno MJ, Moreno-Márquez MDC, Moreno-Alías I, Rapoport H, Fernández-Escobar R (2018) Interaction between mycorrhization with Glomus intraradices and phosphorus in nursery olive plants. Sci Hort 233:249–255. https://doi.org/10.1016/j.scienta.2018.01.057
Kachkouch W, Touati J, Touhami A, Filali-Maltof A, Modafar C, Moukhli A, Oukabli A, Benkirane R, Douira A (2014) Diversity of arbuscular mycorrhizal fungi in the rhizosphere of Olea europaea in three regions of Morocco (Tafilalt, Zagora and Taounate). Int J Pure Appl Biosci 2:178–195
Kapoor R (2008) Induced resistance in mycorrhizal tomato is correlated to concentration of jasmonic acid. J Biol Sci 8:49–56. https://doi.org/10.3844/ojbsci.2008.49.56
Kara Z, Arslan D, Güler M, Güler Ş (2015) Inoculation of arbuscular mycorrhizal fungi and application of micronized calcite to olive plant: effects on some biochemical constituents of olive fruit and oil. Sci Hort 185:219–227. https://doi.org/10.1016/j.scienta.2015.02.001
Lambais MR (2000) Regulation of plant defense-related genes in arbuscular mycorrhizae. In: Podila GK, Douds DD (eds) Current advances in mycorrhizae research. APS Press, Minnesota, pp 45–59
Lambert DH, Cloe H, Baker DE (1980) Variation in the response of Alfalfa clones and cultivars of mycorrhizae and phosphorus. Crop Sci 20:615–618
Liu H, Jiang W, Bi Y, Luo Y (2005) Postharvest BTH treatment induces resistance of peach (Prunus persica L. cv. Jiubao) fruit to infection by Penicillium expansum and enhances activity of fruit defense mechanisms. Postharvest Biol Technol 35:263–269. https://doi.org/10.1016/j.postharvbio.2004.08.006
Long L, Lin Q, Yao Q, Zhu H (2017) Population and function analysis of cultivable bacteria associated with spores of arbuscular mycorrhizal fungus Gigaspora margarita. Biotech 7:8. https://doi.org/10.1007/s13205-017-0612-1
Markakis EA, Tjamos SE, Antoniou PP, Roussos PA, Paplomatas EJ, Tjamos EC (2010) Phenolic responses of resistant and susceptible olive cultivars induced by defoliating and non-defoliating Verticillium dahliae pathotypes. Plant Dis 94:1156–1162. https://doi.org/10.1094/PDIS-94-9-1156
Martos-Moreno C, López-Escudero FJ, Blanco-López MÁ (2006) Resistance of olive cultivars to the defoliating pathotype of Verticillium dahliae. HortScience 41:1313–1316
Mason TBS (2015) Assessing direct and indirect effects of the fungicide Flutriafol on arbuscular mycorrhizal fungi in controlling cotton root rot (Master thesis). In: Texas Tech University, p 88
Morandi D (1996) Occurrence of phytoalexins and phenolic compounds in endomycorrhizal interactions, and their potential role in biological control. Plant Soil 185:241–251
Öpik M, Moora M, Liira J, Zobel M (2006) Composition of root-colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe. J Ecol 94:778–790
Ortega EP, De NB, Coca BM, Torres W, Noval D, Carmona AM, Hernández A, León O (2015) Induction of defense mechanisms in mycorrhized tomato plants against the attack of Oidiopsis taurica (Lev.) Salm. Cultivos Trop 36:94–102
Ouarraqi EM, Oihabi A, Benkhaled L, El Modafar C (2005) Rôle des champignons ectomycorhiziens dans l’induction des mécanismes de défense du Pin d’Alep vis-à-vis de Fusarium oxysporum. Acta Bot Gall 152:77–89. https://doi.org/10.1080/12538078.2005.10515456
Pellegrino E, Bedini S (2014) Enhancing ecosystem services in sustainable agriculture: biofertilization and biofortification of chickpea (Cicer arietinum L.) by arbuscular mycorrhizal fungi. Soil Biol Biochem 68:429–439
Pellegrino E, Bedini S, Avio L, Bonari E, Giovannetti M (2011) Field inoculation effectiveness of native and exotic arbuscular mycorrhizal fungi in a Mediterranean agricultural soil. Soil Biol Biochem 43:367–376
Pomar F, Novo M, Bernal MA, Merino F, Barceló AR (2004) Changes in stem lignins (monomer composition and crosslinking) and peroxidase are related with the maintenance of leaf photosynthetic integrity during Verticillium wilt in Capsicum annuum. New Phytol 163:111–123. https://doi.org/10.1111/j.1469-8137.2004.01092.x
Pozo MJ, Verhage A, García-Andrade J, García JM, Azcón-Aguilar C (2009) Priming plant defence against pathogens by arbuscular mycorrhizal fungi. In: Mycorrhizas-functional processes and ecological impact, pp 123–135
Pusztahelyi T, Holb IJ, Pócsi I (2015) Secondary metabolites in fungus-plant interactions. Front Plant Sci 6:573. https://doi.org/10.3389/fpls.2015.00573
Rahioui B, Aissam S, Messaouri H, Moukhli A, Khadari B, El Modafar C (2013) Role of phenolic metabolism in the defense of the olive-tree against leaf-spot disease caused by Spilocaea oleagina. Int J Agric Biol 15:273–278
Saldajeno MGB, Chandanie WA, Kubota M, Hyakumachi M (2008) Effects of interactions of arbuscular mycorrhizal fungi and beneficial saprophytic mycoflora on plant growth and disease protection. In: Siddiqui ZA, Akhtar MS, Futai K (eds) Mycorrhizae: sustainable agriculture and forestry. Springer, Dordrecht, pp 211–226
Sanei SJ, Razavi SE (2017) Resistance and vegetative growth analysis of some olive cultivars in response to a defoliating pathotype of Verticillium dahliae Kleb. Int J Hortic Sci Technol 4:239–250. https://doi.org/10.22059/ijhst.2018.241208.206
Schwartz MW, Hoeksema JD, Gehring CA, Johnson NC, Klironomos JN, Abbott LK, Pringle A (2006) The promise and the potential consequences of the global transport of mycorrhizal fungal inoculum. Ecol Lett 9:501–515
Serrhini MN, Zeroual A (1995) Verticillium wilt in Morocco. Olivae 58:58–61
Singh M (2015) Interactions among arbuscular mycorrhizal fungi, Trichoderma harzianum, Aspergillus niger and biocontrol of wilt of tomato. Arch Phytopathol Plant Protect 48:205–211. https://doi.org/10.1080/03235408.2014.884825
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol 299:152–178. https://doi.org/10.1016/S0076-6879(99)99017-1
Tarraf W, Ruta C, Tagarelli A, De Cillis F, De Mastro G (2017) Influence of arbuscular mycorrhizae on plant growth, essential oil production and phosphorus uptake of Salvia officinalis L. Ind Crops Prod 102:144–153. https://doi.org/10.1016/j.indcrop.2017.03.010
Wehner J, Antunes PM, Powell JR, Mazukatow J, Rillig MC (2010) Plant pathogen protection by arbuscular mycorrhizas: a role for fungal diversity? Pedobiologia 53:197–201. https://doi.org/10.1016/j.pedobi.2009.10.002
Xu L, Zhu L, Tu L, Liu L, Yuan D, Jin L, Long L, Zhang X (2011) Lignin metabolism has a central role in the resistance of cotton to the wilt fungus Verticillium dahliae as revealed by RNA-Seq-dependent transcriptional analysis and histochemistry. J Exp Bot 62:5607–5621. https://doi.org/10.1093/jxb/err245
Zeng RS (2006) Disease resistance in plants through mycorrhizal fungi induced allelochemicals. In: Inderjit Mukerji KG (ed) Allelochemicals: biological control of plant pathogens and diseases. Springer, Dordrecht, pp 181–192
Acknowledgements
This work was supported by the project ArimNet “Pestolive” and the project “Rhizolive” funded by the “Académie Hassan II des Sciences et Techniques,” Morocco.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boutaj, H., Chakhchar, A., Meddich, A. et al. Mycorrhizal autochthonous consortium induced defense-related mechanisms of olive trees against Verticillium dahliae. J Plant Dis Prot 128, 225–237 (2021). https://doi.org/10.1007/s41348-020-00365-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-020-00365-3