Abstract
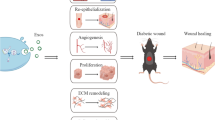
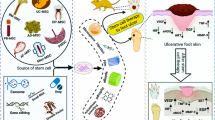
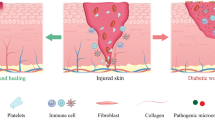
Diabetic foot ischemia and ulcer (DFU) persists as a serious diabetes mellitus complication in spite of increased understanding of the pathophysiology and the cellular and molecular responses. Contributing to this pessimistic situation is the lack of effective treatments that are slow to heal the deep chronic wounds and microvascular obstruction. Mesenchymal stromal cells (MSCs) have been tested as a promising cell-based therapy for diabetes in vitro and in vivo, which is able to accelerate wound closure with increased epithelialization, granulation tissue formation and angiogenesis by differentiation into skin cells and paracrine pathways to repair injured cells. The secretomes of MSCs, including cytokines, growth factors, chemokines, and extracellular vesicles containing mRNA, proteins and microRNAs, have immunomodulatory and regenerative effects. This review will shed new light on the therapeutic potential of MSC-derived extracellular vesicles (MSC-EVs) for the treatment of diabetes-induced lower limb ischemia and ulcers. The identification of underlying mechanisms for MSC-EVs regulation on impaired diabetic wound healing might provide a new direction for MSC-centered treatment for diabetic lower limb ischemia and ulcers.

Immunomodulatory and angiogenic effects of MSC-derived extracellular vesicles on diabetic foot ulcer.

Similar content being viewed by others
References
Internation Diabetes Federation. IDF Diabetes Atlas Ninth. Dunia : IDF (2019).
Cerf, M. E. (2013). Beta cell dysfunction and insulin resistance. Front. Endocrinol. (Lausanne)., 4, 1–12.
Yazdanpanah, L. (2015). Literature review on the management of diabetic foot ulcer. World Journal of Diabetes, 6, 37–53.
Armstrong, D. G., Boulton, A. J. M., & Bus, S. A. (2017). Diabetic foot ulcers and their recurrence. The New England Journal of Medicine, 376, 2367–2375.
Andrew, J. (2005). M Boulton, Loretta Vileikyte, Gunnel Ragnarson-Tennvall. J. A. The global burden of diabetic foot diseas. Lancet, 366, 1719–1724.
Walsh, J. W., Hoffstad, O. J., Sullivan, M. O., & Margolis, D. J. (2016). Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabetic Medicine, 33, 1493–1498.
Shiekh, P. A., Singh, A., & Kumar, A. (2020). Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials, 249, 120020.
Hart, T., Milner, R., & Cifu, A. (2017). Management of a diabetic foot. JAMA - J. Am. Med. Assoc., 318, 1387–1388.
Lee, H. C., An, S. G., Lee, H. W., Park, J. S., Cha, K. S., Hong, T. J., Park, J. H., Lee, S. Y., Kim, S. P., Kim, Y. D., Chung, S. W., Bae, Y. C., Shin, Y. B., Kim, J. I., & Jung, J. S. (2012). Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: - a pilot study. Circulation Journal, 76, 1750–1760.
Mathew, S. A., Naik, C., Cahill, P. A., & Bhonde, R. R. (2019). Placental mesenchymal stromal cells as an alternative tool for therapeutic angiogenesis. Cellular and Molecular Life Sciences, 77, 253–265. https://doi.org/10.1007/s00018-019-03268-1.
Gao, W., Chen, D., Liu, G., & Ran, X. (2019). Autologous stem cell therapy for peripheral arterial disease: A systematic review and meta-analysis of randomized controlled trials. Stem Cell Research & Therapy, 10, 1–14.
Gu, J., Huang, L., Zhang, C., Wang, Y., Zhang, R., Tu, Z., Wang, H., Zhou, X., Xiao, Z., Liu, Z., Hu, X., Ke, Z., Wang, D., & Liu, L. (2020). Therapeutic evidence of umbilical cord-derived mesenchymal stem cell transplantation for cerebral palsy: A randomized, controlled trial. Stem Cell Research & Therapy, 11, 43.
Dalirfardouei, R., Jamialahmadi, K., Jafarian, A. H., & Mahdipour, E. (2019). Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. Journal of Tissue Engineering and Regenerative Medicine, 13, 555–568.
Huang, Y.-Z., Gou, M., Da, L.-C., Zhang, W.-Q., & Xie, H.-Q. (2020). Mesenchymal stem cells for chronic wound healing: current status of preclinical and clinical studies. Tissue Eng. Part B Rev., 1–114. https://doi.org/10.1089/ten.teb.2019.0351.
Cao, Y., Gang, X., Sun, C., & Wang, G. (2017). Mesenchymal stem cells improve healing of diabetic foot ulcer. Journal Diabetes Research, 2017, 1–10.
Li, Q., Zhang, A., Tao, C., Li, X., & Jin, P. (2013). The role of SDF-1-CXCR4/CXCR7 axis in biological behaviors of adipose tissue-derived mesenchymal stem cells in vitro. Biochemical and Biophysical Research Communications, 441, 675–680.
Prütz, W. A., & Mönig, H. (1987). Human Adult CD34_ Progenitor Cells Functionally Express the Chemokine Receptors CCR1, CCR4, CCR7, CXCR5, and CCR10 but Not CXCR4. Int. J. Radiat. Biol. Relat. Stud. Physics, Chem. Med., 52, 677–682.
Segers, V. F. M., et al. (2006). Mesenchymal stem cell adhesion to cardiac microvascular endothelium: Activators and mechanisms. Am. J. Physiol. - Hear. Circ. Physiol., 290, 1370–1377.
Steingen, C., Brenig, F., Baumgartner, L., Schmidt, J., Schmidt, A., & Bloch, W. (2008). Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. Journal of Molecular and Cellular Cardiology, 44, 1072–1084.
Fukuda, T., & Ohnishi, Y. (1991). MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of. Acta Pathologica Japonica, 41, 466–472.
Kim, H. K. et al. A subset of paracrine factors as efficient biomarkers for predicting vascular regenerative efficacy of Mesenchymal stromal/stem cells. Stem Cells 37, 77–88 (2019).
Kato, Y., Iwata, T., Morikawa, S., Yamato, M., Okano, T., & Uchigata, Y. (2015). Allogeneic transplantation of an adipose-derived stem cell sheet combined with artificial skin accelerates wound healing in a rat wound model of type 2 diabetes and obesity. Diabetes, 64, 2723–2734.
Shrestha, C., Zhao, L., Chen, K., He, H., & Mo, Z. (2013). Enhanced healing of diabetic wounds by subcutaneous administration of human umbilical cord derived stem cells and their conditioned media. International Journal of Endocrinology, 2013, 1–10.
You, H. J., Namgoong, S., Han, S. K., Jeong, S. H., Dhong, E. S., & Kim, W. K. (2015). Wound-healing potential of human umbilical cord blood-derived mesenchymal stromal cells in vitro-a pilot study. Cytotherapy, 17, 1506–1513.
Ma, D., Kua, J. E. H., Lim, W. K., Lee, S. T., & Chua, A. W. C. (2015). In vitro characterization of human hair follicle dermal sheath mesenchymal stromal cells and their potential in enhancing diabetic wound healing. Cytotherapy, 17, 1036–1051.
Progenitor, T. (2015) T ISSUE -S PECIFIC P ROGENITOR AND S TEM C ELLS Therapeutic Potential of Adipose-Derived SSEA-3-Positive Muse Cells for Treating Diabetic Skin Ulcers. 146–155.
Cao, Y., Gang, X., Sun, C., & Wang, G. (2017). Mesenchymal stem cells improve healing of diabetic foot ulcer. Journal Diabetes Research, 2017, 1–10.
Navone, S. E., et al. (2014). Decellularized silk fibroin scaffold primed with adipose mesenchymal stromal cells improves wound healing in diabetic mice. Stem Cell Research & Therapy, 5, 1–15.
Zhao, Q. S., Xia, N., Zhao, N., Li, M., Bi, C. L., Zhu, Q., Qiao, G. F., & Cheng, Z. F. (2013). Localization of human mesenchymal stem cells from umbilical cord blood and their role in repair of diabetic foot ulcers in rats. International Journal of Biological Sciences, 10, 80–89.
Xia, N., Xu, J. M., Zhao, N., Zhao, Q. S., Li, M., & Cheng, Z. F. (2015). Human mesenchymal stem cells improve the neurodegeneration of femoral nerve in a diabetic foot ulceration rats. Neuroscience Letters, 597, 84–89.
An, R., et al. (2020). Adipose stem cells isolated from diabetic mice improve cutaneous wound healing in streptozotocin-induced diabetic mice. Stem Cell Research & Therapy, 11, 1–11.
Marfia, G., Navone, S. E., di Vito, C., Ughi, N., Tabano, S., Miozzo, M., Tremolada, C., Bolla, G., Crotti, C., Ingegnoli, F., Rampini, P., Riboni, L., Gualtierotti, R., & Campanella, R. (2015). Mesenchymal stem cells: Potential for therapy and treatment of chronic non-healing skin wounds. Organogenesis, 11, 183–206.
Zhang, Q. Z., Su, W. R., Shi, S. H., Wilder-Smith, P., Xiang, A. P., Wong, A., Nguyen, A. L., Kwon, C. W., & le, A. D. (2010). Human gingiva-derived mesenchymal stem cells elicit polarization of M2 macrophages and enhance cutaneous wound healing. Stem Cells, 28, 1856–1868.
Tong, C., Hao, H., Xia, L., Liu, J., Ti, D., Dong, L., Hou, Q., Song, H., Liu, H., Zhao, Y., Fu, X., & Han, W. (2016). Hypoxia pretreatment of bone marrow - derived mesenchymal stem cells seeded in a collagen-chitosan sponge scaffold promotes skin wound healing in diabetic rats with hindlimb ischemia. Wound Repair and Regeneration, 24, 45–56.
Wu, Y., Huang, S., Enhe, J., Ma, K., Yang, S., Sun, T., & Fu, X. (2014). Bone marrow-derived mesenchymal stem cell attenuates skin fibrosis development in mice. International Wound Journal, 11, 701–710.
Klopp, A. H., Gupta, A., Spaeth, E., Andreeff, M., & Marini, F. (2011). Concise review: Dissecting a discrepancy in the literature: Do mesenchymal stem cells support or suppress tumor growth? Stem Cells, 29, 11–19.
Prockop, D. J., Brenner, M., Fibbe, W. E., Horwitz, E., le Blanc, K., Phinney, D. G., Simmons, P. J., Sensebe, L., & Keating, A. (2010). Defining the risks of mesenchymal stromal cell therapy. Cytotherapy, 12, 576–578.
Ankrum, J. A., Ong, J. F., & Karp, J. M. (2014). Mesenchymal stem cells: Immune evasive, not immune privileged. Nature Biotechnology, 32, 252–260.
Lötvall, J., et al. (2014). Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. Journal of Extracellular Vesicles, 3, 1–6.
Théry, C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., Ayre D.C., Bach J.M., Bachurski D., Baharvand H., Balaj L., Baldacchino S., Bauer N.N., Baxter A.A., Bebawy M., Beckham C., Bedina Zavec A., Benmoussa A., Berardi A.C., Bergese P., Bielska E., Blenkiron C., Bobis-Wozowicz S., Boilard E., Boireau W., Bongiovanni A., Borràs F.E., Bosch S., Boulanger C.M., Breakefield X., Breglio A.M., Brennan M.Á., Brigstock D.R., Brisson A., Broekman M.L.D., Bromberg J.F., Bryl-Górecka P., Buch S., Buck A.H., Burger D., Busatto S., Buschmann D., Bussolati B., Buzás E.I., Byrd J.B., Camussi G., Carter D.R.F., Caruso S., Chamley L.W., Chang Y.T., Chen C., Chen S., Cheng L., Chin A.R., Clayton A., Clerici S.P., Cocks A., Cocucci E., Coffey R.J., Cordeiro-da-Silva A., Couch Y., Coumans F.A.W., Coyle B., Crescitelli R., Criado M.F., D’Souza-Schorey C., Das S., Datta Chaudhuri A., de Candia P., de Santana Junior E.F., de Wever O., del Portillo H.A., Demaret T., Deville S., Devitt A., Dhondt B., di Vizio D., Dieterich L.C., Dolo V., Dominguez Rubio A.P., Dominici M., Dourado M.R., Driedonks T.A.P., Duarte F.V., Duncan H.M., Eichenberger R.M., Ekström K., el Andaloussi S., Elie-Caille C., Erdbrügger U., Falcón-Pérez J.M., Fatima F., Fish J.E., Flores-Bellver M., Försönits A., Frelet-Barrand A., Fricke F., Fuhrmann G., Gabrielsson S., Gámez-Valero A., Gardiner C., Gärtner K., Gaudin R., Gho Y.S., Giebel B., Gilbert C., Gimona M., Giusti I., Goberdhan D.C.I., Görgens A., Gorski S.M., Greening D.W., Gross J.C., Gualerzi A., Gupta G.N., Gustafson D., Handberg A., Haraszti R.A., Harrison P., Hegyesi H., Hendrix A., Hill A.F., Hochberg F.H., Hoffmann K.F., Holder B., Holthofer H., Hosseinkhani B., Hu G., Huang Y., Huber V., Hunt S., Ibrahim A.G.E., Ikezu T., Inal J.M., Isin M., Ivanova A., Jackson H.K., Jacobsen S., Jay S.M., Jayachandran M., Jenster G., Jiang L., Johnson S.M., Jones J.C., Jong A., Jovanovic-Talisman T., Jung S., Kalluri R., Kano S.I., Kaur S., Kawamura Y., Keller E.T., Khamari D., Khomyakova E., Khvorova A., Kierulf P., Kim K.P., Kislinger T., Klingeborn M., Klinke II D.J., Kornek M., Kosanović M.M., Kovács Á.F., Krämer-Albers E.M., Krasemann S., Krause M., Kurochkin I.V., Kusuma G.D., Kuypers S., Laitinen S., Langevin S.M., Languino L.R., Lannigan J., Lässer C., Laurent L.C., Lavieu G., Lázaro-Ibáñez E., le Lay S., Lee M.S., Lee Y.X.F., Lemos D.S., Lenassi M., Leszczynska A., Li I.T.S., Liao K., Libregts S.F., Ligeti E., Lim R., Lim S.K., Linē A., Linnemannstöns K., Llorente A., Lombard C.A., Lorenowicz M.J., Lörincz Á.M., Lötvall J., Lovett J., Lowry M.C., Loyer X., Lu Q., Lukomska B., Lunavat T.R., Maas S.L.N., Malhi H., Marcilla A., Mariani J., Mariscal J., Martens-Uzunova E.S., Martin-Jaular L., Martinez M.C., Martins V.R., Mathieu M., Mathivanan S., Maugeri M., McGinnis L.K., McVey M.J., Meckes Jr D.G., Meehan K.L., Mertens I., Minciacchi V.R., Möller A., Møller Jørgensen M., Morales-Kastresana A., Morhayim J., Mullier F., Muraca M., Musante L., Mussack V., Muth D.C., Myburgh K.H., Najrana T., Nawaz M., Nazarenko I., Nejsum P., Neri C., Neri T., Nieuwland R., Nimrichter L., Nolan J.P., Nolte-’t Hoen E.N.M., Noren Hooten N., O’Driscoll L., O’Grady T., O’Loghlen A., Ochiya T., Olivier M., Ortiz A., Ortiz L.A., Osteikoetxea X., Østergaard O., Ostrowski M., Park J., Pegtel D.M., Peinado H., Perut F., Pfaffl M.W., Phinney D.G., Pieters B.C.H., Pink R.C., Pisetsky D.S., Pogge von Strandmann E., Polakovicova I., Poon I.K.H., Powell B.H., Prada I., Pulliam L., Quesenberry P., Radeghieri A., Raffai R.L., Raimondo S., Rak J., Ramirez M.I., Raposo G., Rayyan M.S., Regev-Rudzki N., Ricklefs F.L., Robbins P.D., Roberts D.D., Rodrigues S.C., Rohde E., Rome S., Rouschop K.M.A., Rughetti A., Russell A.E., Saá P., Sahoo S., Salas-Huenuleo E., Sánchez C., Saugstad J.A., Saul M.J., Schiffelers R.M., Schneider R., Schøyen T.H., Scott A., Shahaj E., Sharma S., Shatnyeva O., Shekari F., Shelke G.V., Shetty A.K., Shiba K., Siljander P.R.M., Silva A.M., Skowronek A., Snyder II O.L., Soares R.P., Sódar B.W., Soekmadji C., Sotillo J., Stahl P.D., Stoorvogel W., Stott S.L., Strasser E.F., Swift S., Tahara H., Tewari M., Timms K., Tiwari S., Tixeira R., Tkach M., Toh W.S., Tomasini R., Torrecilhas A.C., Tosar J.P., Toxavidis V., Urbanelli L., Vader P., van Balkom B.W.M., van der Grein S.G., van Deun J., van Herwijnen M.J.C., van Keuren-Jensen K., van Niel G., van Royen M.E., van Wijnen A.J., Vasconcelos M.H., Vechetti Jr I.J., Veit T.D., Vella L.J., Velot É., Verweij F.J., Vestad B., Viñas J.L., Visnovitz T., Vukman K.V., Wahlgren J., Watson D.C., Wauben M.H.M., Weaver A., Webber J.P., Weber V., Wehman A.M., Weiss D.J., Welsh J.A., Wendt S., Wheelock A.M., Wiener Z., Witte L., Wolfram J., Xagorari A., Xander P., Xu J., Yan X., Yáñez-Mó M., Yin H., Yuana Y., Zappulli V., Zarubova J., Žėkas V., Zhang J.Y., Zhao Z., Zheng L., Zheutlin A.R., Zickler A.M., Zimmermann P., Zivkovic A.M., Zocco D., Zuba-Surma E.K. (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7.
Di Liegro, C. M., Schiera, G. & Di Liegro, I. (2017) Extracellular vesicle-associated RNA as a carrier of epigenetic information. Genes (Basel) 8.
Lee, Y., El Andaloussi, S., & Wood, M. J. A. (2012). Exosomes and microvesicles: Extracellular vesicles for genetic information transfer and gene therapy. Human Molecular Genetics, 21, 125–134.
Gardiner, C., et al. (2016). Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. J. Extracell. Vesicles, 5, 1–6.
Momen-Heravi, F., Balaj, L., Alian, S., Mantel, P. Y., Halleck, A. E., Trachtenberg, A. J., Soria, C. E., Oquin, S., Bonebreak, C. M., Saracoglu, E., Skog, J., & Kuo, W. P. (2013). Current methods for the isolation of extracellular vesicles. Biological Chemistry, 394, 1253–1262.
Konoshenko, M. Y., Lekchnov, E. A., Vlassov, A. V., & Laktionov, P. P. (2018). Isolation of extracellular vesicles: General methodologies and latest trends. BioMed Research International, 2018, 1–27.
Benedikter, B. J., et al. (2017). Ultrafiltration combined with size exclusion chromatography efficiently isolates extracellular vesicles from cell culture media for compositional and functional studies. Scientific Reports, 7, 1–13.
Fafián-Labora, J., Morente-López, M., Sánchez-Dopico, M. J., Arntz, O. J., van de Loo, F. A. J., de Toro, J., & Arufe, M. C. (2020). Influence of mesenchymal stem cell-derived extracellular vesicles in vitro and their role in ageing. Stem Cell Research & Therapy, 11, 13.
Wu, P., Zhang, B., Shi, H., Qian, H., & Xu, W. (2018). MSC-exosome: A novel cell-free therapy for cutaneous regeneration. Cytotherapy, 20, 291–301.
Casado-Díaz, A., Quesada-Gómez, J. M., & Dorado, G. (2020). Extracellular vesicles derived from Mesenchymal stem cells (MSC) in regenerative medicine: Applications in skin wound healing. Frontiers in Bioengineering and Biotechnology, 8, 1–19.
Zhang, D., Xuan, J., Zheng, B. B., Zhou, Y. L., Lin, Y., Wu, Y. S., Zhou, Y. F., Huang, Y. X., Wang, Q., Shen, L. Y., Mao, C., Wu, Y., Wang, X. Y., Tian, N. F., Xu, H. Z., & Zhang, X. L. (2017). Metformin improves functional recovery after spinal cord injury via autophagy flux stimulation. Molecular Neurobiology, 54, 3327–3341.
Madhyastha, R., Madhyastha, H. K., Pengjam, Y., Nakajima, Y., Omura, S., & Maruyama, M. (2014). NFkappaB activation is essential for miR-21 induction by TGFβ1 in high glucose conditions. Biochemical and Biophysical Research Communications, 451, 615–621.
Hu, Y., Rao, S. S., Wang, Z. X., Cao, J., Tan, Y. J., Luo, J., Li, H. M., Zhang, W. S., Chen, C. Y., & Xie, H. (2018). Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics, 8, 169–184.
Zhou, Q., Gallagher, R., Ufret-Vincenty, R., Li, X., Olson, E. N., & Wang, S. (2011). Regulation of angiogenesis and choroidal neovascularization by members of microRNA-23∼27∼24 clusters. Proceedings of the National Academy of Sciences of the United States of America, 108, 8287–8292.
Huang, F., et al. (2013). Mesenchymal stem cells modified with miR-126 release angiogenic factors and activate notch ligand Delta-like-4, enhancing ischemic angiogenesis and cell survival. International Journal of Molecular Medicine, 31, 484–492.
Geiger, A., Walker, A., & Nissen, E. (2015). Human fibrocyte-derived exosomes accelerate wound healing in genetically diabetic mice. Biochemical and Biophysical Research Communications, 467, 303–309.
Wang, S., Aurora, A. B., Johnson, B. A., Qi, X., McAnally, J., Hill, J. A., Richardson, J. A., Bassel-Duby, R., & Olson, E. N. (2008). The endothelial-specific MicroRNA miR-126 governs vascular integrity and angiogenesis. Developmental Cell, 15, 261–271.
Tao, S.-C., Guo, S. C., Li, M., Ke, Q. F., Guo, Y. P., & Zhang, C. Q. (2017). Chitosan wound dressings incorporating Exosomes derived from MicroRNA-126-overexpressing Synovium Mesenchymal stem cells provide sustained release of Exosomes and heal full-thickness skin defects in a diabetic rat model. Stem Cells Translational Medicine, 6, 736–747.
Zhang, N., Geng T., Wang Z., Zhang R., Cao T., Camporez J.P., Cai S.Y., Liu Y., Dandolo L., Shulman G.I., Carmichael G.G., Taylor H.S., Huang Y. (2018) Elevated hepatic expression of H19 long noncoding RNA contributes to diabetic hyperglycemia. JCI insight 3.
Ti, D., et al. (2015). LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. Journal of Translational Medicine, 13, 1–14.
Yang, Z. G., Awan, F. M., du, W. W., Zeng, Y., Lyu, J., Wu, D., Gupta, S., Yang, W., & Yang, B. B. (2017). The circular RNA interacts with STAT3, increasing its nuclear translocation and wound repair by modulating Dnmt3a and miR-17 function. Molecular Therapy, 25, 2062–2074.
Bianchetti, L., Barczyk, M., Cardoso, J., Schmidt, M., Bellini, A., & Mattoli, S. (2012). Extracellular matrix remodelling properties of human fibrocytes. Journal of Cellular and Molecular Medicine, 16, 483–495.
He, L. et al. ADSC - Exos containing MALAT1 promotes wound healing by targeting miR - 124 through activating Wnt / β - catenin pathway. Biosci Rep. 2020;40(5)BSR20192549. doihttps://doi.org/10.1042/BSR20192549.
Mollenhauer, J., et al. (2000). DMBT1 encodes a protein involved in the immune defense and in epithelial differentiation and is highly unstable in cancer. Cancer Research, 60, 1704–1710.
Das, A., Ganesh, K., Khanna, S., Sen, C. K., & Roy, S. (2014). Engulfment of apoptotic cells by macrophages: A role of MicroRNA-21 in the resolution of wound inflammation. Journal of Immunology, 192, 1120–1129.
Chen, C. Y., Rao, S. S., Ren, L., Hu, X. K., Tan, Y. J., Hu, Y., Luo, J., Liu, Y. W., Yin, H., Huang, J., Cao, J., Wang, Z. X., Liu, Z. Z., Liu, H. M., Tang, S. Y., Xu, R., & Xie, H. (2018). Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics, 8, 1607–1623.
Shiekh, P. A., Singh, A., & Kumar, A. (2020). Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials, 249, 120020.
Ma, Q. (2015) Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 401–426 doi:https://doi.org/10.1146/annurev-pharmtox-011112-140320.Role.
Li, X., Xie, X., Lian, W., Shi, R., Han, S., Zhang, H., Lu, L., & Li, M. (2018). Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Experimental & Molecular Medicine, 50, 29.
Tsuji, K., Kitamura, S., & Wada, J. (2020). Immunomodulatory and regenerative effects of mesenchymal stem cell-derived extracellular vesicles in renal diseases. International Journal of Molecular Sciences, 21.
Han, Z., Chen, Y., Zhang, Y., Wei, A., Zhou, J., Li, Q., & Guo, L. (2017). MiR-21/PTEN Axis promotes skin wound healing by dendritic cells enhancement. Journal of Cellular Biochemistry, 118, 3511–3519.
Amin, K. N., et al. (2020). miR-23c regulates wound healing by targeting stromal cell-derived factor-1α (SDF-1α/CXCL12) among patients with diabetic foot ulcer. Microvasc. Res, 127, 103924.
Fang, S., Xu, C., Zhang, Y., Xue, C., Yang, C., Bi, H., Qian, X., Wu, M., Ji, K., Zhao, Y., Wang, Y., Liu, H., & Xing, X. (2016). Umbilical cord-derived Mesenchymal stem cell-derived Exosomal MicroRNAs suppress Myofibroblast differentiation by inhibiting the transforming growth factor-beta/SMAD2 pathway during wound healing. Stem Cells Translational Medicine, 5, 1425–1439.
Chen, J. J., & Zhou, S. H. (2011). Mesenchymal stem cells overexpressing MiR-126 enhance ischemic angiogenesis via the AKT/ERK-related pathway. Cardiology Journal, 18, 675–681.
Hu, J., Zeng, L., Huang, J., Wang, G., & Lu, H. (2015). MiR-126 promotes angiogenesis and attenuates inflammation after contusion spinal cord injury in rats. Brain Research, 1608, 191–202.
Fish, J. E., et al. (2008). miR-126 regulates Angiogenic signaling and vascular integrity. Dev Cell, 15, 272–284.
Li, B., Luan, S., Chen, J., Zhou, Y., Wang, T., Li, Z., Fu, Y., Zhai, A., & Bi, C. (2020). The MSC-derived Exosomal lncRNA H19 promotes wound healing in diabetic foot ulcers by Upregulating PTEN via MicroRNA-152-3p. Mol. Ther. - Nucleic Acids, 19, 814–826.
Shi, R., Jin, Y., Hu, W., Lian, W., Cao, C., Han, S., Zhao, S., Yuan, H., Yang, X., Shi, J., & Zhao, H. (2020). Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am. J. Physiol. Physiol., 318, C848–C856.
Choi, Y. H., Burdick, M. D., & Strieter, R. M. (2010). Human circulating fibrocytes have the capacity to differentiate osteoblasts and chondrocytes. The International Journal of Biochemistry & Cell Biology, 42, 662–671.
Pachler, K., Lener, T., Streif, D., Dunai, Z. A., Desgeorges, A., Feichtner, M., Öller, M., Schallmoser, K., Rohde, E., & Gimona, M. (2017). A good manufacturing practice–grade standard protocol for exclusively human mesenchymal stromal cell–derived extracellular vesicles. Cytotherapy, 19, 458–472.
Mendt, M., et al. (2018). Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI insight, 3, 1–22.
Funding
This study was supported by grants from Health Science and Medical technology of Zhejiang Province (2020KY344).
Author information
Authors and Affiliations
Contributions
Tao An: Conceptualization, Tables and diagram, Writing- Original draft preparation.
Yi Chen: Syntax checking. Yingchun Tu: Syntax checking.
Ping Lin: Writing- Reviewing and Editing.
Corresponding author
Ethics declarations
Competing Interests
No financial or nonfinancial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Additional information
Guest Editor: Giovanni Camussi
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Special Issue on Exosomes and Microvesicles: from Stem Cell Biology to Translation in Human Diseases
Rights and permissions
About this article
Cite this article
An, T., Chen, Y., Tu, Y. et al. Mesenchymal Stromal Cell-Derived Extracellular Vesicles in the Treatment of Diabetic Foot Ulcers: Application and Challenges. Stem Cell Rev and Rep 17, 369–378 (2021). https://doi.org/10.1007/s12015-020-10014-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-020-10014-9