Abstract
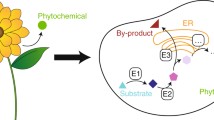
Microbial fermentation platforms offer a cost-effective and sustainable alternative to plant cultivation and chemical synthesis for the production of many plant-derived pharmaceuticals. Plant alkaloids, particularly benzylisoquinoline alkaloids and monoterpene indole alkaloids, and recently cannabinoids have become attractive targets for microbial biosynthesis owing to their medicinal importance. Recent advances in the discovery of pathway components, together with the application of synthetic biology tools, have facilitated the assembly of plant alkaloid and cannabinoid pathways in the microbial hosts Escherichia coli and Saccharomyces cerevisiae. This review highlights key aspects of these pathways in the framework of overcoming bottlenecks in microbial production to further improve end-product titers. We discuss the opportunities that emerge from a better understanding of the pathway components by further study of the plant, and strategies for generation of new and advanced medicinal compounds.





Similar content being viewed by others
References
Newman DJ, Cragg GM (2020) Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod 83:770–803. https://doi.org/10.1021/acs.jnatprod.9b01285
World Health Organization (2019) WHO model list of essential medicines, 21th list
Martino E, Casamassima G, Castiglione S et al (2018) Vinca alkaloids and analogues as anti-cancer agents: looking back, peering ahead. Bioorg Med Chem Lett 28:2816–2826. https://doi.org/10.1016/j.bmcl.2018.06.044
Andre CM, Hausman J-F, Guerriero G (2016) Cannabis sativa: the plant of the thousand and one molecules. Front Plant Sci 7:19
Paddon CJ, Keasling JD (2014) Semi-synthetic artemisinin: a model for the use of synthetic biology in pharmaceutical development. Nat Rev Microbiol 12:355–367. https://doi.org/10.1038/nrmicro3240
Singh A, Menéndez-Perdomo IM, Facchini PJ (2019) Benzylisoquinoline alkaloid biosynthesis in opium poppy: an update. Phytochem Rev 18:1457–1482. https://doi.org/10.1007/s11101-019-09644-w
Pyne ME, Narcross L, Martin VJJ (2019) Engineering plant secondary metabolism in microbial systems. Plant Physiol 179:844–861. https://doi.org/10.1104/pp.18.01291
Cravens A, Payne J, Smolke CD (2019) Synthetic biology strategies for microbial biosynthesis of plant natural products. Nat Commun 10:1–12. https://doi.org/10.1038/s41467-019-09848-w
Chen R, Yang S, Zhang L, Zhou YJ (2020) Advanced strategies for production of natural products in yeast. iScience 23:100879. https://doi.org/10.1016/j.isci.2020.100879
Birchfield AS, McIntosh CA (2020) Metabolic engineering and synthetic biology of plant natural products—a minireview. Curr Plant Biol. https://doi.org/10.1016/j.cpb.2020.100163
Facchini PJ, Park S-U (2003) Developmental and inducible accumulation of gene transcripts involved in alkaloid biosynthesis in opium poppy. Phytochemistry 64:177–186. https://doi.org/10.1016/S0031-9422(03)00292-9
Ounaroon A, Decker G, Schmidt J et al (2003) (R,S)-Reticuline 7-O-methyltransferase and (R,S)-norcoclaurine 6-O-methyltransferase of Papaver somniferum – cDNA cloning and characterization of methyl transfer enzymes of alkaloid biosynthesis in opium poppy. Plant J 36:808–819. https://doi.org/10.1046/j.1365-313X.2003.01928.x
Frick S, Kramell R, Kutchan TM (2007) Metabolic engineering with a morphine biosynthetic P450 in opium poppy surpasses breeding. Metab Eng 9:169–176. https://doi.org/10.1016/j.ymben.2006.10.004
Ziegler J, Diaz-Chávez ML, Kramell R et al (2005) Comparative macroarray analysis of morphine containing Papaver somniferum and eight morphine free Papaver species identifies an O-methyltransferase involved in benzylisoquinoline biosynthesis. Planta 222:458–471. https://doi.org/10.1007/s00425-005-1550-4
Farrow SC, Hagel JM, Beaudoin GAW et al (2015) Stereochemical inversion of (S)-reticuline by a cytochrome P450 fusion in opium poppy. Nat Chem Biol 11:728–732. https://doi.org/10.1038/nchembio.1879
Winzer T, Kern M, King AJ et al (2015) Morphinan biosynthesis in opium poppy requires a P450-oxidoreductase fusion protein. Science 349:309–312. https://doi.org/10.1126/science.aab1852
Gesell A, Rolf M, Ziegler J et al (2009) CYP719B1 is salutaridine synthase, the C-C phenol-coupling enzyme of morphine biosynthesis in opium poppy. J Biol Chem 284:24432–24442. https://doi.org/10.1074/jbc.M109.033373
Ziegler J, Voigtländer S, Schmidt J et al (2006) Comparative transcript and alkaloid profiling in Papaver species identifies a short chain dehydrogenase/reductase involved in morphine biosynthesis. Plant J 48:177–192. https://doi.org/10.1111/j.1365-313X.2006.02860.x
Lenz R, Zenk MH (1995) Acetyl coenzyme A:salutaridinol-7-O-acetyltransferase from Papaver somniferum plant cell cultures. J Biol Chem 270:31091–31096. https://doi.org/10.1074/jbc.270.52.31091
Grothe T, Lenz R, Kutchan TM (2001) Molecular characterization of the salutaridinol 7-O-Acetyltransferase involved in morphine biosynthesis in opium poppy Papaver somniferum. J Biol Chem 276:30717–30723. https://doi.org/10.1074/jbc.M102688200
Chen X, Hagel JM, Chang L et al (2018) A pathogenesis-related 10 protein catalyzes the final step in thebaine biosynthesis article. Nat Chem Biol 14:738–743. https://doi.org/10.1038/s41589-018-0059-7
Lee E-J, Facchini P (2010) Norcoclaurine synthase is a member of the pathogenesis-related 10/Bet v1 protein family. Plant Cell 22:3489–3503. https://doi.org/10.1105/tpc.110.077958
Hagel JM, Facchini PJ (2010) Dioxygenases catalyze the O-demethylation steps of morphine biosynthesis in opium poppy. Nat Chem Biol 6:273–275. https://doi.org/10.1038/nchembio.317
Unterlinner B, Lenz R, Kutchan TM (1999) Molecular cloning and functional expression of codeinone reductase: the penultimate enzyme in morphine biosynthesis in the opium poppy Papaver somniferum. Plant J 18:465–475. https://doi.org/10.1046/j.1365-313X.1999.00470.x
Dastmalchi M, Chen X, Hagel JM et al (2019) Neopinone isomerase is involved in codeine and morphine biosynthesis in opium poppy. Nat Chem Biol 15:384–390. https://doi.org/10.1038/s41589-019-0247-0
Dastmalchi M, Chang L, Torres MA et al (2018) Codeinone reductase isoforms with differential stability, efficiency and product selectivity in opium poppy. Plant J 95:631–647. https://doi.org/10.1111/tpj.13975
Thodey K, Galanie S, Smolke CD (2014) A microbial biomanufacturing platform for natural and semisynthetic opioids. Nat Chem Biol 10:837–844. https://doi.org/10.1038/nchembio.1613
Galanie S, Thodey K, Trenchard IJ et al (2015) Complete biosynthesis of opioids in yeast. Science 349:1095–1100. https://doi.org/10.1126/science.aac9373
Brown S, Clastre M, Courdavault V, O’Connor SE (2015) De novo production of the plant-derived alkaloid strictosidine in yeast. Proc Natl Acad Sci USA 112:3205–3210. https://doi.org/10.1073/pnas.1423555112
Qu Y, Easson MEAM, Simionescu R et al (2018) Solution of the multistep pathway for assembly of corynanthean, strychnos, iboga, and aspidosperma monoterpenoid indole alkaloids from 19E-geissoschizine. Proc Natl Acad Sci USA 115:3180–3185. https://doi.org/10.1073/pnas.1719979115
Qu Y, Easson MLAE, Froese J et al (2015) Completion of the seven-step pathway from tabersonine to the anticancer drug precursor vindoline and its assembly in yeast. Proc Natl Acad Sci USA 112:6224–6229. https://doi.org/10.1073/PNAS.1501821112
Qu Y, Thamm AMK, Czerwinski M et al (2018) Geissoschizine synthase controls flux in the formation of monoterpenoid indole alkaloids in a Catharanthus roseus mutant. Planta 247:625–634. https://doi.org/10.1007/s00425-017-2812-7
Caputi L, Franke J, Farrow SC et al (2018) Missing enzymes in the biosynthesis of the anticancer drug vinblastine in Madagascar periwinkle. Science 360:1235–1239. https://doi.org/10.1126/science.aat4100
Qu Y, Safonova O, De Luca V (2019) Completion of the canonical pathway for assembly of anticancer drugs vincristine/vinblastine in Catharanthus roseus. Plant J 97:257–266. https://doi.org/10.1111/tpj.14111
Taura F, Tanaka S, Taguchi C et al (2009) Characterization of olivetol synthase, a polyketide synthase putatively involved in cannabinoid biosynthetic pathway. FEBS Lett 583:2061–2066
Gagne SJ, Stout JM, Liu E et al (2012) Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides. Proc Natl Acad Sci USA 109:12811–12816. https://doi.org/10.1073/pnas.1200330109
Sirikantaramas S, Taura F, Tanaka Y et al (2005) Tetrahydrocannabinolic acid synthase, the enzyme controlling marijuana psychoactivity, is secreted into the storage cavity of the glandular trichomes. Plant Cell Physiol 46:1578–1582. https://doi.org/10.1093/pcp/pci166
Taura F, Morimoto S, Shoyama Y (1996) Purification and characterization of cannabidiolic-acid synthase from Cannabis sativa L. Biochemical analysis of a novel enzyme that catalyzes the oxidocyclization of cannabigerolic acid to cannabidiolic acid. J Biol Chem 271:17411–17416. https://doi.org/10.1074/jbc.271.29.17411
Taura F, Sirikantaramas S, Shoyama Y et al (2007) Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa. FEBS Lett 581:2929–2934. https://doi.org/10.1016/j.febslet.2007.05.043
Luo X, Reiter MA, d’Espaux L et al (2019) Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature 567:123–126. https://doi.org/10.1038/s41586-019-0978-9
Zirpel B, Degenhardt F, Martin C et al (2017) Engineering yeasts as platform organisms for cannabinoid biosynthesis. J Biotechnol 259:204–212. https://doi.org/10.1016/j.jbiotec.2017.07.008
Dastmalchi M, Chang L, Chen R et al (2019) Purine permease-type benzylisoquinoline alkaloid transporters in opium poppy. Plant Physiol 181:916–933. https://doi.org/10.1104/pp.19.00565
Fossati E, Ekins A, Narcross L et al (2014) Reconstitution of a 10-gene pathway for synthesis of the plant alkaloid dihydrosanguinarine in Saccharomyces cerevisiae. Nat Commun 5:3283. https://doi.org/10.1038/ncomms4283
Yu F, De Luca V (2013) ATP-binding cassette transporter controls leaf surface secretion of anticancer drug components in Catharanthus roseus. Proc Natl Acad Sci USA 110:15830–15835. https://doi.org/10.1073/pnas.1307504110
Payne RME, Xu D, Foureau E et al (2017) An NPF transporter exports a central monoterpene indole alkaloid intermediate from the vacuole. Nat Plants 3:16208. https://doi.org/10.1038/nplants.2016.208
Larsen B, Fuller VL, Pollier J et al (2017) Identification of iridoid glucoside transporters in Catharanthus roseus. Plant Cell Physiol 58:1507–1518. https://doi.org/10.1093/pcp/pcx097
Livingston SJ, Quilichini TD, Booth JK et al (2020) Cannabis glandular trichomes alter morphology and metabolite content during flower maturation. Plant J 101:37–56. https://doi.org/10.1111/tpj.14516
Hwang J-U, Song W-Y, Hong D et al (2016) Plant ABC transporters enable many unique aspects of a terrestrial plant’s lifestyle. Mol Plant 9:338–355. https://doi.org/10.1016/j.molp.2016.02.003
Laverty KU, Stout JM, Sullivan MJ et al (2019) A physical and genetic map of Cannabis sativa identifies extensive rearrangements at the THC/CBD acid synthase loci. Genome Res 29:146–156. https://doi.org/10.1101/gr.242594.118
Kellner F, Kim J, Clavijo BJ et al (2015) Genome-guided investigation of plant natural product biosynthesis. Plant J 82:680–692. https://doi.org/10.1111/tpj.12827
Dugé de Bernonville T, Foureau E, Parage C et al (2015) Characterization of a second secologanin synthase isoform producing both secologanin and secoxyloganin allows enhanced de novo assembly of a Catharanthus roseus transcriptome. BMC Genomics 16:619. https://doi.org/10.1186/s12864-015-1678-y
Munkert J, Pollier J, Miettinen K et al (2015) Iridoid synthase activity is common among the plant progesterone 5β-reductase family. Mol Plant 8:136–152. https://doi.org/10.1016/j.molp.2014.11.005
Besseau S, Kellner F, Lanoue A et al (2013) A pair of tabersonine 16-hydroxylases initiates the synthesis of vindoline in an organ-dependent manner in Catharanthus roseus. Plant Physiol 163:1792–1803. https://doi.org/10.1104/pp.113.222828
DeLoache WC, Russ ZN, Narcross L et al (2015) An enzyme-coupled biosensor enables (S)-reticuline production in yeast from glucose. Nat Chem Biol 11:465–471. https://doi.org/10.1038/nchembio.1816
Runguphan W, Glenn WS, O’Connor SE (2012) Redesign of a dioxygenase in morphine biosynthesis. Chem Biol 19:674–678. https://doi.org/10.1016/j.chembiol.2012.04.017
Denby CM, Li RA, Vu VT et al (2018) Industrial brewing yeast engineered for the production of primary flavor determinants in hopped beer. Nat Commun 9:965. https://doi.org/10.1038/s41467-018-03293-x
Ro D-K, Paradise EM, Ouellet M et al (2006) Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440:940–943. https://doi.org/10.1038/nature04640
Paddon CJ, Westfall PJ, Pitera DJ et al (2013) High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 496:528–532. https://doi.org/10.1038/nature12051
Campbell A, Bauchart P, Gold ND et al (2016) Engineering of a nepetalactol-producing platform strain of Saccharomyces cerevisiae for the production of plant seco-iridoids. ACS Synth Biol 5:405–414. https://doi.org/10.1021/acssynbio.5b00289
Luttik MAH, Vuralhan Z, Suir E et al (2008) Alleviation of feedback inhibition in Saccharomyces cerevisiae aromatic amino acid biosynthesis: quantification of metabolic impact. Metab Eng 10:141–153. https://doi.org/10.1016/j.ymben.2008.02.002
Trenchard IJ, Siddiqui MS, Thodey K, Smolke CD (2015) De novo production of the key branch point benzylisoquinoline alkaloid reticuline in yeast. Metab Eng 31:74–83. https://doi.org/10.1016/j.ymben.2015.06.010
Kluza A, Niedzialkowska E, Kurpiewska K et al (2018) Crystal structure of thebaine 6-O-demethylase from the morphine biosynthesis pathway. J Struct Biol 202:229–235. https://doi.org/10.1016/j.jsb.2018.01.007
Shoyama Y, Tamada T, Kurihara K et al (2012) Structure and function of ∆1-tetrahydrocannabinolic acid (THCA) synthase, the enzyme controlling the psychoactivity of Cannabis sativa. J Mol Biol 423:96–105. https://doi.org/10.1016/j.jmb.2012.06.030
Ma X, Panjikar S, Koepke J et al (2006) The structure of Rauvolfia serpentina strictosidine synthase is a novel six-bladed β-propeller fold in plant proteins. Plant Cell 18:907–920. https://doi.org/10.1105/tpc.105.038018
Bennett MR, Thompson ML, Shepherd SA et al (2018) Structure and biocatalytic scope of coclaurine N-methyltransferase. Angew Chemie Int Ed 57:10600–10604. https://doi.org/10.1002/anie.201805060
Lang DE, Morris JS, Rowley M et al (2019) Structure-function studies of tetrahydroprotoberberine N-methyltransferase reveal the molecular basis of stereoselective substrate recognition. J Biol Chem 294:14482–14898. https://doi.org/10.1074/jbc.RA119.009214
French CE, Bruce NC (1994) Purification and characterization of morphinone reductase from Pseudomonas putida M10. Biochem J 301:97–103. https://doi.org/10.1042/bj3010097
French CE, Hailes AM, Rathbone DA et al (1995) Biological production of semisynthetic opiates using genetically engineered bacteria. Bio/Technology 13:674–676. https://doi.org/10.1038/nbt0795-674
Nakagawa A, Matsumura E, Koyanagi T et al (2016) Total biosynthesis of opiates by stepwise fermentation using engineered Escherichia coli. Nat Commun 7:10390. https://doi.org/10.1038/ncomms10390
Nakagawa A, Matsuzaki C, Matsumura E et al (2014) (R, S)-Tetrahydropapaveroline production by stepwise fermentation using engineered Escherichia coli. Sci Rep 4:6695. https://doi.org/10.1038/srep06695
Lütke-Eversloh T, Stephanopoulos G (2007) L-Tyrosine production by deregulated strains of Escherichia coli. Appl Microbiol Biotechnol 75:103–110. https://doi.org/10.1007/s00253-006-0792-9
Minami H, Kim J-S, Ikezawa N et al (2008) Microbial production of plant benzylisoquinoline alkaloids. Proc Natl Acad Sci USA 105:7393–7398. https://doi.org/10.1073/pnas.0802981105
Nakagawa A, Minami H, Kim J-S et al (2011) A bacterial platform for fermentative production of plant alkaloids. Nat Commun 2:326. https://doi.org/10.1038/ncomms1327
Kim J-S, Nakagawa A, Yamazaki Y et al (2013) Improvement of reticuline productivity from dopamine by using engineered Escherichia coli. Biosci Biotechnol Biochem 77:2166–2168. https://doi.org/10.1271/bbb.130552
Matsumura E, Nakagawa A, Tomabechi Y et al (2017) Laboratory-scale production of (S)-reticuline, an important intermediate of benzylisoquinoline alkaloids, using a bacterial-based method. Biosci Biotechnol Biochem 81:396–402. https://doi.org/10.1080/09168451.2016.1243985
Carey JS, Laffan D, Thomson C, Williams MT (2006) Analysis of the reactions used for the preparation of drug candidate molecules. Org Biomol Chem 4:2337–2347. https://doi.org/10.1039/B602413K
Neumann CS, Fujimori DG, Walsh CT (2008) Halogenation strategies in natural product biosynthesis. Chem Biol 15:99–109. https://doi.org/10.1016/j.chembiol.2008.01.006
Li Y, Li S, Thodey K et al (2018) Complete biosynthesis of noscapine and halogenated alkaloids in yeast. Proc Natl Acad Sci USA 115:3922–3931. https://doi.org/10.1073/pnas.1721469115
McCoy E, O’Connor SE (2006) Directed biosynthesis of alkaloid analogs in the medicinal plant Catharanthus roseus. J Am Chem Soc 128:14276–14277. https://doi.org/10.1021/ja066787w
Runguphan W, Qu X, O’Connor SE (2010) Integrating carbon–halogen bond formation into medicinal plant metabolism. Nature 468:461–464. https://doi.org/10.1038/nature09524
Markey SP (2007) CHAPTER 11-Pathways of drug metabolism. In: Abernethy DR, Daniels CE, et al. (eds) Atkinson AJ. Academic Press, Burlington, pp 143–162
Matsumura E, Nakagawa A, Tomabechi Y et al (2018) Microbial production of novel sulphated alkaloids for drug discovery. Sci Rep 8:7980. https://doi.org/10.1038/s41598-018-26306-7
Pyne ME, Kevvai K, Grewal PS et al (2020) A yeast platform for high-level synthesis of tetrahydroisoquinoline alkaloids. Nat Commun 11:3337. https://doi.org/10.1038/s41467-020-17172-x
Bow EW, Rimoldi JM (2016) The structure–function relationships of classical cannabinoids: CB1/CB2 modulation. Perspect Medicin Chem 8:17–39. https://doi.org/10.4137/PMC.S32171
Zipp BJ, Hardman JM BR (2017) Cannabinoid glycoside prodrugs and methods of synthesis. WO2017053574A1.
Acknowledgements
We thank Jillian Hagel for a critical manuscript review. This work was supported by an Alberta Applied Agricultural Genomics Program grant from Genome Alberta (project number A3GP16) and a Strategic Project Grant from Alberta Innovates (project number G2018000889) to PJF.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ozber, N., Watkins, J.L. & Facchini, P.J. Back to the plant: overcoming roadblocks to the microbial production of pharmaceutically important plant natural products. J Ind Microbiol Biotechnol 47, 815–828 (2020). https://doi.org/10.1007/s10295-020-02300-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-020-02300-9