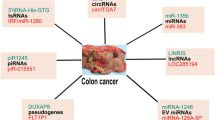
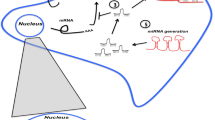
Abstract
More than 50% of colorectal cancer (CRC) deaths are attributed to metastasis, and the liver is the most common distant metastatic site of CRC. The molecular mechanisms underlying CRC liver metastasis are very complicated and remain largely unknown. Accumulated evidence has shown that non-coding RNAs (NcRNAs) play critical roles in tumor development and progression. Here we reviewed the roles and underlying mechanisms of NcRNAs in CRC liver metastasis.
Similar content being viewed by others
Data availability
Not applicable.
References
Ferlay J, Shin H-R, Bray F et al (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917. https://doi.org/10.1002/ijc.25516
Scholefield JH, Steele RJ (2002) Guidelines for follow up after resection of colorectal cancer. Gut 51(Suppl 5):V3–5. https://doi.org/10.1136/gut.51.suppl_5.v3
Yamashita S, Chun YS, Kopetz SE, Vauthey J-N (2018) Biomarkers in colorectal liver metastases. Br J Surg 105:618–627. https://doi.org/10.1002/bjs.10834
Tsitskari M, Filippiadis D, Kostantos C et al (2019) The role of interventional oncology in the treatment of colorectal cancer liver metastases. Ann Gastroenterol 32:147–155. https://doi.org/10.20524/aog.2018.0338
Van Cutsem E, Cervantes A, Adam R et al (2016) ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol Off J Eur Soc Med Oncol 27:1386–1422. https://doi.org/10.1093/annonc/mdw235
Wang J-P, Tang Y-Y, Fan C-M et al (2018) The role of exosomal non-coding RNAs in cancer metastasis. Oncotarget 9:12487–12502. https://doi.org/10.18632/oncotarget.23552
Klingenberg M, Matsuda A, Diederichs S, Patel T (2017) Non-coding RNA in hepatocellular carcinoma: mechanisms, biomarkers and therapeutic targets. J Hepatol 67:603–618. https://doi.org/10.1016/j.jhep.2017.04.009
Li P-F, Chen S-C, Xia T et al (2014) Non-coding RNAs and gastric cancer. World J Gastroenterol 20:5411–5419. https://doi.org/10.3748/wjg.v20.i18.5411
Zhang X, Ma X, Jing S et al (2018) Non-coding RNAs and retroviruses. Retrovirology 15:20. https://doi.org/10.1186/s12977-018-0403-8
Li P, Ou Q, Braciak TA et al (2018) MicroRNA-192-5p is a predictive biomarker of survival for Stage IIIB colon cancer patients. Jpn J Clin Oncol 48:619–624. https://doi.org/10.1093/jjco/hyy019
Friedman RC, Farh KK-H, Burge CB, Bartel DP (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19:92–105. https://doi.org/10.1101/gr.082701.108
Bartel DP (2018) Metazoan MicroRNAs. Cell 173:20–51. https://doi.org/10.1016/j.cell.2018.03.006
Markopoulos GS, Roupakia E, Tokamani M et al (2017) A step-by-step microRNA guide to cancer development and metastasis. Cell Oncol (Dordr) 40:303–339. https://doi.org/10.1007/s13402-017-0341-9
Xin Z, Ma Q, Ren S et al (2017) The understanding of circular RNAs as special triggers in carcinogenesis. Brief Funct Genomics 16:80–86. https://doi.org/10.1093/bfgp/elw001
Sanger HL, Klotz G, Riesner D et al (1976) Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci USA 73:3852–3856. https://doi.org/10.1073/pnas.73.11.3852
Chen L-L (2016) The biogenesis and emerging roles of circular RNAs. Nat Rev Mol Cell Biol 17:205–211. https://doi.org/10.1038/nrm.2015.32
Hao S, Cong L, Qu R et al (2019) Emerging roles of circular RNAs in colorectal cancer. Onco Targets Ther 12:4765–4777. https://doi.org/10.2147/OTT.S208235
Hansen TB, Jensen TI, Clausen BH et al (2013) Natural RNA circles function as efficient microRNA sponges. Nature 495:384–388. https://doi.org/10.1038/nature11993
Wu J, Qi X, Liu L et al (2019) Emerging epigenetic regulation of circular RNAs in human cancer. Mol Ther Nucleic Acids 16:589–596. https://doi.org/10.1016/j.omtn.2019.04.011
Zhao X, Cai Y, Xu J (2019) Circular RNAs: biogenesis, mechanism, and function in human cancers. Int J Mol Sci. https://doi.org/10.3390/ijms20163926
Wilusz JE (2018) A 360° view of circular RNAs: from biogenesis to functions. Wiley Interdiscip Rev RNA 9:e1478. https://doi.org/10.1002/wrna.1478
Rashid F, Shah A, Shan G (2016) Long non-coding RNAs in the cytoplasm. Genom Proteomics Bioinform 14:73–80. https://doi.org/10.1016/j.gpb.2016.03.005
He J-H, Han Z-P, Li Y-G (2014) Association between long non-coding RNA and human rare diseases (review). Biomed Rep 2:19–23. https://doi.org/10.3892/br.2013.191
Ip JY, Nakagawa S (2012) Long non-coding RNAs in nuclear bodies. Dev Growth Differ 54:44–54. https://doi.org/10.1111/j.1440-169X.2011.01303.x
Dahariya S, Paddibhatla I, Kumar S et al (2019) Long non-coding RNA: classification, biogenesis and functions in blood cells. Mol Immunol 112:82–92. https://doi.org/10.1016/j.molimm.2019.04.011
Wu H, Yang L, Chen L-L (2017) The diversity of long noncoding RNAs and their generation. Trends Genet 33:540–552. https://doi.org/10.1016/j.tig.2017.05.004
Derrien T, Johnson R, Bussotti G et al (2012) The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res 22:1775–1789. https://doi.org/10.1101/gr.132159.111
Wang KC, Chang HY (2011) Molecular mechanisms of long noncoding RNAs. Mol Cell 43:904–914. https://doi.org/10.1016/j.molcel.2011.08.018
He Y, Meng X-M, Huang C et al (2014) Long noncoding RNAs: novel insights into hepatocelluar carcinoma. Cancer Lett 344:20–27. https://doi.org/10.1016/j.canlet.2013.10.021
Rinn JL, Kertesz M, Wang JK et al (2007) Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129:1311–1323. https://doi.org/10.1016/j.cell.2007.05.022
Huarte M, Guttman M, Feldser D et al (2010) A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 142:409–419. https://doi.org/10.1016/j.cell.2010.06.040
Azzalin CM, Reichenbach P, Khoriauli L et al (2007) Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 318:798–801. https://doi.org/10.1126/science.1147182
Nagano T, Mitchell JA, Sanz LA et al (2008) The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science 322:1717–1720. https://doi.org/10.1126/science.1163802
Lee JT (2010) The X as model for RNA’s niche in epigenomic regulation. Cold Spring Harb Perspect Biol 2:a003749. https://doi.org/10.1101/cshperspect.a003749
Kotake Y, Nakagawa T, Kitagawa K et al (2011) Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene 30:1956–1962. https://doi.org/10.1038/onc.2010.568
Pandey RR, Mondal T, Mohammad F et al (2008) Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol Cell 32:232–246. https://doi.org/10.1016/j.molcel.2008.08.022
Wang D, Liu J, Huo T et al (2017) The role of microRNAs in colorectal liver metastasis: important participants and potential clinical significances. Tumour Biol J Int Soc Oncodevelopmental Biol Med 39:1010428317709640. https://doi.org/10.1177/1010428317709640
Mohammadi A, Mansoori B, Baradaran B (2016) The role of microRNAs in colorectal cancer. Biomed Pharmacother 84:705–713. https://doi.org/10.1016/j.biopha.2016.09.099
Ding M, Zhang T, Li S et al (2017) Correlation analysis between liver metastasis and serum levels of miR-200 and miR-141 in patients with colorectal cancer. Mol Med Rep 16:7791–7795. https://doi.org/10.3892/mmr.2017.7538
Yu H, Shen Y, Hong J et al (2015) The contribution of TGF-β in epithelial-mesenchymal transition (EMT): down-regulation of E-cadherin via snail. Neoplasma 62:1–15. https://doi.org/10.4149/neo_2015_002
Liu Y, Zhang Y, Wu H et al (2017) miR-10a suppresses colorectal cancer metastasis by modulating the epithelial-to-mesenchymal transition and anoikis. Cell Death Dis 8:e2739. https://doi.org/10.1038/cddis.2017.61
Li J, Xia L, Zhou Z et al (2018) MiR-186-5p upregulation inhibits proliferation, metastasis and epithelial-to-mesenchymal transition of colorectal cancer cell by targeting ZEB1. Arch Biochem Biophys 640:53–60. https://doi.org/10.1016/j.abb.2018.01.002
Li J, Chen Y, Guo X et al (2016) Inhibition of miR-15b decreases cell migration and metastasis in colorectal cancer. Tumour Biol J Int Soc Oncodevelopmental Biol Med 37:8765–8773. https://doi.org/10.1007/s13277-015-4396-9
Asangani IA, Rasheed SAK, Nikolova DA et al (2008) MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 27:2128–2136. https://doi.org/10.1038/sj.onc.1210856
Zhu W, Luo X, Fu H et al (2019) MiR-3653 inhibits the metastasis and epithelial-mesenchymal transition of colon cancer by targeting Zeb2. Pathol Res Pract 215:152577. https://doi.org/10.1016/j.prp.2019.152577
Hong S, Yan Z, Wang H et al (2019) Up-regulation of microRNA-497-5p inhibits colorectal cancer cell proliferation and invasion via targeting PTPN3. Biosci Rep. https://doi.org/10.1042/BSR20191123
Xie Y, Zhao J, Liang Y et al (2019) MicroRNA-10b controls the metastasis and proliferation of colorectal cancer cells by regulating Krüppel-like factor 4. Artif Cells Nanomed Biotechnol 47:1722–1729. https://doi.org/10.1080/21691401.2019.1606006
Mokutani Y, Uemura M, Munakata K et al (2016) Down-regulation of microRNA-132 is associated with poor prognosis of colorectal cancer. Ann Surg Oncol 23:599–608. https://doi.org/10.1245/s10434-016-5133-3
Chen D-L, Wang Z-Q, Zeng Z-L et al (2014) Identification of microRNA-214 as a negative regulator of colorectal cancer liver metastasis by way of regulation of fibroblast growth factor receptor 1 expression. Hepatology 60:598–609. https://doi.org/10.1002/hep.27118
Bleau A-M, Redrado M, Nistal-Villan E et al (2018) miR-146a targets c-met and abolishes colorectal cancer liver metastasis. Cancer Lett 414:257–267. https://doi.org/10.1016/j.canlet.2017.11.008
Li W, Chang J, Wang S et al (2015) miRNA-99b-5p suppresses liver metastasis of colorectal cancer by down-regulating mTOR. Oncotarget 6:24448–24462. https://doi.org/10.18632/oncotarget.4423
Ji D, Chen Z, Li M et al (2014) MicroRNA-181a promotes tumor growth and liver metastasis in colorectal cancer by targeting the tumor suppressor WIF-1. Mol Cancer 13:86. https://doi.org/10.1186/1476-4598-13-86
Guo L, Fu J, Sun S et al (2019) MicroRNA-143-3p inhibits colorectal cancer metastases by targeting ITGA6 and ASAP3. Cancer Sci 110:805–816. https://doi.org/10.1111/cas.13910
Hata T, Mokutani Y, Takahashi H et al (2017) Identification of microRNA-487b as a negative regulator of liver metastasis by regulation of KRAS in colorectal cancer. Int J Oncol 50:487–496. https://doi.org/10.3892/ijo.2016.3813
Xu X-W, Zheng B-A, Hu Z-M et al (2017) Circular RNA hsa_circ_000984 promotes colon cancer growth and metastasis by sponging miR-106b. Oncotarget 8:91674–91683. https://doi.org/10.18632/oncotarget.21748
Geng Y, Zheng X, Hu W et al (2019) Hsa_circ_0009361 acts as the sponge of miR-582 to suppress colorectal cancer progression by regulating APC2 expression. Clin Sci (Lond) 133:1197–1213. https://doi.org/10.1042/CS20190286
Xu H, Wang C, Song H et al (2019) RNA-Seq profiling of circular RNAs in human colorectal Cancer liver metastasis and the potential biomarkers. Mol Cancer 18:8
Chen L-Y, Zhi Z, Wang L et al (2019) NSD2 circular RNA promotes metastasis of colorectal cancer by targeting miR-199b-5p-mediated DDR1 and JAG1 signalling. J Pathol 248:103–115. https://doi.org/10.1002/path.5238
Yong W, Zhuoqi X, Baocheng W et al (2018) Hsa_circ_0071589 promotes carcinogenesis via the miR-600/EZH2 axis in colorectal cancer. Biomed Pharmacother 102:1188–1194. https://doi.org/10.1016/j.biopha.2018.03.085
Zheng X, Chen L, Zhou Y et al (2019) A novel protein encoded by a circular RNA circPPP1R12A promotes tumor pathogenesis and metastasis of colon cancer via Hippo-YAP signaling. Mol Cancer 18:47. https://doi.org/10.1186/s12943-019-1010-6
Hsiao K-Y, Lin Y-C, Gupta SK et al (2017) Noncoding effects of circular RNA CCDC66 promote colon cancer growth and metastasis. Cancer Res 77:2339–2350. https://doi.org/10.1158/0008-5472.CAN-16-1883
Li X, Wang J, Zhang C et al (2018) Circular RNA circITGA7 inhibits colorectal cancer growth and metastasis by modulating the Ras pathway and upregulating transcription of its host gene ITGA7. J Pathol 246:166–179. https://doi.org/10.1002/path.5125
Chen D-L, Lu Y-X, Zhang J-X et al (2017) Long non-coding RNA UICLM promotes colorectal cancer liver metastasis by acting as a ceRNA for microRNA-215 to regulate ZEB2 expression. Theranostics 7:4836–4849. https://doi.org/10.7150/thno.20942
Ye L, Ren L, Qiu J et al (2015) Aberrant expression of long noncoding RNAs in colorectal cancer with liver metastasis. Tumour Biol J Int Soc Oncodevelopmental Biol Med 36:8747–8754. https://doi.org/10.1007/s13277-015-3627-4
Huang L, Lin H, Kang L et al (2019) Aberrant expression of long noncoding RNA SNHG15 correlates with liver metastasis and poor survival in colorectal cancer. J Cell Physiol 234:7032–7039. https://doi.org/10.1002/jcp.27456
Wang Y, Lu Z, Wang N et al (2018) Long noncoding RNA DANCR promotes colorectal cancer proliferation and metastasis via miR-577 sponging. Exp Mol Med 50:1–17. https://doi.org/10.1038/s12276-018-0082-5
Fellig Y, Ariel I, Ohana P et al (2005) H19 expression in hepatic metastases from a range of human carcinomas. J Clin Pathol 58:1064–1068. https://doi.org/10.1136/jcp.2004.023648
Cai Y, Yan P, Zhang G et al (2018) Long non-coding RNA TP73-AS1 sponges miR-194 to promote colorectal cancer cell proliferation, migration and invasion via up-regulating TGFα. Cancer Biomark 23:145–156. https://doi.org/10.3233/CBM-181503
Di W, Weinan X, Xin L et al (2019) Long noncoding RNA SNHG14 facilitates colorectal cancer metastasis through targeting EZH2-regulated EPHA7. Cell Death Dis 10:514. https://doi.org/10.1038/s41419-019-1707-x
Jia G-Q, Zhang M-M, Wang K et al (2018) Long non-coding RNA PlncRNA-1 promotes cell proliferation and hepatic metastasis in colorectal cancer. J Cell Biochem 119:7091–7104. https://doi.org/10.1002/jcb.27031
Spaderna S, Schmalhofer O, Hlubek F et al (2006) A transient, EMT-linked loss of basement membranes indicates metastasis and poor survival in colorectal cancer. Gastroenterology 131:830–840. https://doi.org/10.1053/j.gastro.2006.06.016
Lin M-T, Song H-J, Ding X-Y (2018) Long non-coding RNAs involved in metastasis of gastric cancer. World J Gastroenterol 24:3724–3737. https://doi.org/10.3748/wjg.v24.i33.3724
Cao H, Xu E, Liu H et al (2015) Epithelial-mesenchymal transition in colorectal cancer metastasis: a system review. Pathol Res Pract 211:557–569. https://doi.org/10.1016/j.prp.2015.05.010
Cui F, Wang S, Lao I et al (2016) miR-375 inhibits the invasion and metastasis of colorectal cancer via targeting SP1 and regulating EMT-associated genes. Oncol Rep 36:487–493. https://doi.org/10.3892/or.2016.4834
Zhu L, Chen H, Zhou D et al (2012) MicroRNA-9 up-regulation is involved in colorectal cancer metastasis via promoting cell motility. Med Oncol 29:1037–1043. https://doi.org/10.1007/s12032-011-9975-z
Shelton PM, Duran A, Nakanishi Y et al (2018) The Secretion of miR-200s by a PKCζ/ADAR2 signaling axis promotes liver metastasis in colorectal cancer. Cell Rep 23:1178–1191. https://doi.org/10.1016/j.celrep.2018.03.118
Chen R-X, Chen X, Xia L-P et al (2019) N(6)-methyladenosine modification of circNSUN2 facilitates cytoplasmic export and stabilizes HMGA2 to promote colorectal liver metastasis. Nat Commun 10:4695. https://doi.org/10.1038/s41467-019-12651-2
Li Y, Zhao Z, Xu C et al (2014) HMGA2 induces transcription factor Slug expression to promote epithelial-to-mesenchymal transition and contributes to colon cancer progression. Cancer Lett 355:130–140. https://doi.org/10.1016/j.canlet.2014.09.007
Fodde R, Brabletz T (2007) Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol 19:150–158. https://doi.org/10.1016/j.ceb.2007.02.007
Tam C, Wong JH, Tsui SKW et al (2019) LncRNAs with miRNAs in regulation of gastric, liver, and colorectal cancers: updates in recent years. Appl Microbiol Biotechnol 103:4649–4677. https://doi.org/10.1007/s00253-019-09837-5
Chen D, Sun Q, Cheng X et al (2016) Genome-wide analysis of long noncoding RNA (lncRNA) expression in colorectal cancer tissues from patients with liver metastasis. Cancer Med 5:1629–1639. https://doi.org/10.1002/cam4.738
Khatri VP, Petrelli NJ, Belghiti J (2005) Extending the frontiers of surgical therapy for hepatic colorectal metastases: is there a limit? J Clin Oncol Off J Am Soc Clin Oncol 23:8490–8499. https://doi.org/10.1200/JCO.2004.00.6155
Van Schaeybroeck S, Allen WL, Turkington RC, Johnston PG (2011) Implementing prognostic and predictive biomarkers in CRC clinical trials. Nat Rev Clin Oncol 8:222–232. https://doi.org/10.1038/nrclinonc.2011.15
Oshima G, Guo N, He C et al (2017) In vivo delivery and therapeutic effects of a microRNA on colorectal liver metastases. Mol Ther 25:1588–1595. https://doi.org/10.1016/j.ymthe.2017.04.005
Jiang T, Ye L, Han Z et al (2017) miR-19b-3p promotes colon cancer proliferation and oxaliplatin-based chemoresistance by targeting SMAD4: validation by bioinformatics and experimental analyses. J Exp Clin Cancer Res 36:131. https://doi.org/10.1186/s13046-017-0602-5
Wang Q, Shi L, Shi K et al (2020) CircCSPP1 functions as a ceRNA to promote colorectal carcinoma cell EMT and liver metastasis by upregulating COL1A1. Front Oncol 10:850. https://doi.org/10.3389/fonc.2020.00850
Shan Y, Ma J, Pan Y et al (2018) LncRNA SNHG7 sponges miR-216b to promote proliferation and liver metastasis of colorectal cancer through upregulating GALNT1. Cell Death Dis 9:722. https://doi.org/10.1038/s41419-018-0759-7
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81572424), Natural Science Foundation of Guangdong Province (Grant No. 2017A030313482), Guangzhou Municipal Science and Technology Project (Grant No. 201607010164), Scientific Research Project of Guangzhou Municipal Colleges and Universities (Grant No. 1201610322).
Author information
Authors and Affiliations
Contributions
X-YZ finished the manuscript and abstract; BL and Z-KJ consulted relevant literatures and completed English revision; Y-KX and F-CW completed the figures and tables; J-QH provided constructive feedback and guidance; J-SC completed critical revisions and proofread the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no potential conflicts of interest.
Consent for publication
All authors agree to publish the article.
Ethical approval and consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, XY., Luo, B., Jiang, ZK. et al. Non-coding RNAS and colorectal cancer liver metastasis. Mol Cell Biochem 475, 151–159 (2020). https://doi.org/10.1007/s11010-020-03867-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03867-8