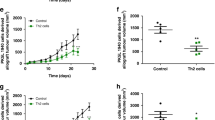
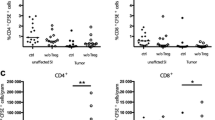
Abstract
Regulatory T cells (Tregs) and transforming growth factor β (TGF-β) are believed to play key roles in both postoperative pro-inflammatory and anti-inflammatory responses of malignancies. Recombinant human thrombomodulin (rTM) is implied to inhibit the interaction between TGF-β and Tregs. The aim of this study is to evaluate the antitumor effects of rTM against gastrointestinal tumors under systemic inflammation. Mice were subjected to cecal ligation and puncture and percutaneous allogeneic tumor implantation. rTM were introduced by percutaneous injection into the abdominal cavity. The effects of rTM were evaluated by weight of implanted tumor, proportion of Tregs in peripheral blood lymphocytes (PBL) and tumor infiltrating lymphocytes (TIL) and temporal evaluation of serum cytokines. The effect of rTM was also evaluated on the in vitro differentiation of naïve T cells into induced Tregs induced by TGF-β and interleukin (IL) -2. rTM significantly inhibited the proliferation of the implanted tumor cells in an inflammation-dependent manner. rTM also reduced the fractions of regulatory T cells and induced regulatory T cells among both PBL and TIL. Temporal evaluation of serum cytokine levels in the model mice showed that rTM significantly suppressed the increases in the serum levels of IL-2 and TGF-β. An in vitro differentiation assay revealed that rTM inhibited the differentiation of naïve T cells into Tregs triggered by IL-2- and TGF-β. rTM has suppressive effects on inflammation-induced gastrointestinal tumor growth by suggestively affecting differentiation of Tregs.





Similar content being viewed by others
Data availability
Cell line: CT26.CL25 cell line, ATCC (RRID: CVCL_7255). Mouse: BALB/cCrSlc mice (RRID: MGI:2161077). Software: FACSDiva v8.0.1 (RRID: SCR_001456). SPSS (RRID:SCR_002865). Antibody: CD4 (D702Z) rabbit mAb (RRID: AB_2798898). Foxp3 (D608R) rabbit mAb (RRID: AB_2797979). CD8α (D4W2Z) XP rabbit mAb (AB_2756376). Anti-Mouse CD4 FITC (RM4-5)) (RRID: AB_464902). Anti-Mouse CD25 APC (PC61.5) (RRID: AB_469366). Anti-Mouse/Rat Foxp3 PE (FJK-16s) (RRID: AB_469978). Anti-Mouse CD125-APC-Vio770 (RRID: AB_2654795). Anti-mouse CD62L PE-Cy™7 (RRID: AB_394182). Murine anti-TGF-beta 1 antibody (RRID: AB_10562492).
All data generated or analyzed during this study are included in this published article.
References
Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M (2013) Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol 20(5):1575–1583
Kataoka K, Takeuchi H, Mizusawa J, Igaki H, Ozawa S, Abe T et al (2017) Prognostic impact of postoperative morbidity after esophagectomy for esophageal cancer: exploratory analysis of JCOG9907. Ann Surg 265(6):1152–1157
International Surgical Outcomes Study Group (2016) Global patient outcomes after elective surgery: prospective cohort study in 27 low-, middle- and high-income countries. Br J Anaesth 117(5):601–609
Vogel TR, Dombrovskiy VY, Lowry SF (2012) Impact of infectious complications after elective surgery on hospital readmission and late deaths in the US. Medicare Popul 13(5):307–311
Novotny AR, Reim D, Assfalg V, Altmayr F, Friess HM, Emmanuel K et al (2012) Mixed antagonist response and sepsis severity-dependent dysbalance of pro- and anti-inflammatory responses at the onset of postoperative sepsis. Immunobiology 217(6):616–621
Chen W (2011) Tregs in immunotherapy: opportunities and challenges. Immunotherapy 3(8):911–914
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA (2013) Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer 13(11):759–771
Buras JA, Holzmann B, Sitkovsky M (2005) Animal models of sepsis: setting the stage. Nat Rev Drug Discov 4(10):854–865
Toscano MG, Ganea D, Gamero AM (2011) Cecal ligation puncture procedure. J Vis Exp 51:e2860
Wichterman KA, Baue AE, Chaudry IH (1980) Sepsis and septic shock—a review of laboratory models and a proposal. J Surg Res 29(2):189–201
Suda K, Takeuchi H, Hagiwara T, Miyasho T, Okamoto M, Kawasako K et al (2010) Neutrophil elastase inhibitor improves survival of rats with clinically relevant sepsis. Shock 33(5):526–531
Suda K, Kitagawa Y, Ozawa S, Saikawa Y, Ueda M, Ebina M et al (2006) Anti-high-mobility group box chromosomal protein 1 antibodies improve survival of rats with sepsis. World J Surg 30(9):1755–1762
Ogawa H, Yonezawa S, Maruyama I, Matsushita Y, Tezuka Y, Toyoyama H et al (2000) Expression of thrombomodulin in squamous cell carcinoma of the lung: its relationship to lymph node metastasis and prognosis of the patients. Cancer Lett 149(1–2):95–103
Hanly AM, Redmond M, Winter DC, Brophy S, Deasy JM, Bouchier-Hayes DJ et al (2006) Thrombomodulin expression in colorectal carcinoma is protective and correlates with survival. Br J Cancer 94(9):1320–1325
Pathak R, Wang J, Garg S, Aykin-Burns N, Petersen KU, Hauer-Jensen M (2016) Recombinant thrombomodulin (Solulin) ameliorates early intestinal radiation toxicity in a preclinical rat model. Radiat Res 186(2):112–120
Akita N, Ma N, Okamoto T, Asanuma K, Yoshida K, Nishioka J et al (2015) Host protein C inhibitor inhibits tumor growth, but promotes tumor metastasis, which is closely correlated with hypercoagulability. Thromb Res 135(6):1203–1208
Hall BM (2015) T cells: soldiers and spies—the surveillance and control of effector T cells by regulatory T cells. Clin J Am Soc Nephrol 10(11):2050–2064
Gerberick GF, Cruse LW, Miller CM, Sikorski EE, Ridder GM (1997) Selective modulation of T cell memory markers CD62L and CD44 on murine draining lymph node cells following allergen and irritant treatment. Toxicol Appl Pharmacol 146(1):1–10
Hsu P, Santner-Nanan B, Hu M, Skarratt K, Lee CH, Stormon M et al (2015) IL-10 Potentiates differentiation of human induced regulatory T cells via STAT3 and Foxo1. J Immunol 195(8):3665–3674
Hall BM, Plain KM, Tran GT, Verma ND, Robinson CM, Nomura M et al (2017) Cytokines affecting CD4(+)T regulatory cells in transplant tolerance. III. Interleukin-5 (IL-5) promotes survival of alloantigen-specific CD4(+) T regulatory cells. Transpl Immunol 43–44:33–41
Joyce DE, Grinnell BW (2002) Recombinant human activated protein C attenuates the inflammatory response in endothelium and monocytes by modulating nuclear factor-kappaB. Crit Care Med 30(5 Suppl):S288–S293
Esmon CT (2012) Protein C anticoagulant system–anti-inflammatory effects. Semin Immunopathol 34(1):127–132
Yu H, Kortylewski M, Pardoll D (2007) Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 7(1):41–51
Rodrigues M, Griffith LG, Wells AJSCR (2010) Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cells Res Ther 1(4):32
Lin C-R, Wei T-YW, Tsai H-Y, Wu Y-T, Wu P-Y, Chen S-T (2015) Glycosylation-dependent interaction between CD69 and S100A8/S100A9 complex is required for regulatory T-cell differentiation. The FASEB J 29(12):5006–5017
Davies M, Robinson M, Smith E, Huntley S, Prime S, Paterson I (2005) Induction of an epithelial to mesenchymal transition in human immortal and malignant keratinocytes by TGF-beta1 involves MAPK, Smad and AP-1 signalling pathways. J Cell Biochem 95(5):918–931
Sandusky G, Berg DT, Richardson MA, Myers L, Grinnell BW (2002) Modulation of thrombomodulin-dependent activation of human protein C through differential expression of endothelial Smads. J Biol Chem 277(51):49815–49819
Yu S, Wang Y, Hou J, Li W, Wang X, Xiang L et al (2020) Tumor-infiltrating immune cells in hepatocellular carcinoma: Tregs is correlated with poor overall survival. PLoS ONE 15(4):e0231003
Brummel-Ziedins K, Mann KG (2018) Chapter 126—molecular basis of blood coagulation. In: Hoffman R, Benz EJ, Silberstein LE, Heslop HE, Weitz JI, Anastasi J, et al. (eds) Hematology, 7th edn. Elsevier, New York, pp 1885–1905
Funding
En Amada, Kazumasa Fukuda, Koshi Kumagai and Hirofumi Kawakubo received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Yuko Kitagawa was funded by AsahiKASEI Co., Ltd., TAIHO PHARMACEUTICAL CO., LTD, CHUGAI PHARMACEUTICAL CO., LTD., DAIICHI SANKYO COMPANY, LIMITED, Merck Serono Co., Ltd., EA Pharma Co., Ltd., Yakult Honsha Co. Ltd., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Factory Inc., SHIONOGI & CO., LTD., KAKEN PHARMACEUTICAL CO.,LTD., Kowa Pharmaceutical Co., Ltd., Astellas Pharma Inc., MEDICON INC., DAINIPPON SUMITOMO PHARMA Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Kyouwa Hakkou Kirin Co., Ltd., Pfizer Japan Inc., ONO PHARMACEUTICAL CO., LTD., NIHON PHARMACEUTICAL CO., LTD., Japan Blood Products Organization, Medtronic Japan Co., Ltd., Sanofi K.K., grants from Eisai Co., Ltd., TSUMURA & CO., KCI Licensing, Inc., ABBOTT JAPAN CO., LTD., and FUJIFILM Toyama Chemical Co., Ltd.
Author information
Authors and Affiliations
Contributions
EA designed the experiments, performed the experiments, analyzed the data and drafted the manuscript. KF and KK participated in the coordination of study and designing of the experiments. HK and YK participated in interpretation and collection of data. All authors have approved the final version of the manuscript and are accountable for all aspects of this study.
Corresponding author
Ethics declarations
Conflict of interest
En Amada, Kazumasa Fukuda, Koshi Kumagai and Hirofumi Kawakubo declared no potential conflicts of interest with respect to the research, authorship, or publication of this article. Yuko Kitagawa has received compound of recombinant thrombomodulin (rTM) from Asahi Kasei Pharma, Tokyo, Japan.
Ethical approval
All animal procedures were conducted in accordance with Institutional Guidelines on Animal Experimentation of Keio University and were approved by the ethics committee (approval number: 18077-(0)). All staff that participated in the study received special training in animal care and handling from the Laboratory Animal Center of Keio University of Medicine.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
AMADA, E., Fukuda, K., Kumagai, K. et al. Soluble recombinant human thrombomodulin suppresses inflammation-induced gastrointestinal tumor growth in a murine peritonitis model. Mol Cell Biochem 475, 195–203 (2020). https://doi.org/10.1007/s11010-020-03872-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03872-x