An Overview of Cadmium, Chromium, and Lead Content in Bivalves Consumed by the Community of Santa Rosa Island (Ecuador) and Its Health Risk Assessment
- 1Centro de Estudios Aplicados en Química, Pontificia Universidad Católica del Ecuador, Quito, Ecuador
- 2“Ciudad Abierta” Project in Architecture College, Pontificia Universidad Católica del Ecuador, Quito, Ecuador
- 3Environmental Management Career, Pontificia Universidad Católica del Ecuador, Esmeraldas, Ecuador
- 4Marine Biology Career, Pontificia Universidad Católica del Ecuador, Portoviejo, Ecuador
Santa Rosa Island community members derive their income and livelihoods from bio-aquatic resources, principally bivalves of the genus Anadara, both for subsistence use and commercial purposes. Bivalve mollusks have a sedentary lifestyle and feed by filtering water, meaning they absorb all surrounding substances, including harmful elements like toxic metals. This study aimed to analyze different-sized samples of Anadara tuberculosa and Anadara similis, sediment, and Rhizophora mangle leaves to determine their total amount of cadmium, lead, and chromium as a first approach to the evaluation of the health risk related to the consumption of bivalves. For both species from four sampling sites, the results revealed metal concentrations in the bivalves between 0.211 and 0.948 mg kg–1, 0.038, and 0.730 mg kg–1, and 0.067 and 0.923 mg⋅kg–1 for Cd, Cr, and Pb, respectively. The calculated potential risk (>1) for cadmium, considering all body weights, showed a high health risk for consumers. In the case of lead, the results showed a high health risk in children. There was no risk found for chromium. For sediments, the mean values were 2.14, 29.99, and 12.37 mg⋅kg–1 and for the Rhizophora mangle leaves were 2.23, 4.22, and 3.35 mg⋅kg–1 for Cd, Cr, and Pb, respectively. These results did not show a relation with the metal content in bivalves.
Introduction
Over the past decades, anthropogenic activities resulting from, urban and agricultural industrialization development, and waste disposal, have led to the increase of chemical pollution in coastal and marine ecosystems (da Silveira Fiori et al., 2018; Esposito et al., 2018). Coastal zones with river inflows are the most affected due to the continuous drag of contaminants, which constantly accumulate in marine sediments and organisms. Thus, marine organisms such as bivalves that inhabit the coasts are more likely to be exposed to high levels of contaminants, including trace metals. Hence, they represent a threat to human health when used for consumption because of their bioaccumulation and biomagnification through the aquatic food web (Jiang et al., 2018).
Mangroves are one of the most complex ecosystems in the world and are located in tropical and subtropical regions of terrestrial environments, estuaries, and near marine coasts. They are unique biomes that serve as a transition from the terrestrial to the marine environment. While there are no accurate estimates of the original cover, there is a consensus that it would have been over 200,000 km2 and that considerably more than 50,000 km2 or one-quarter of original mangrove cover has been lost because of human intervention (Spalding et al., 2010).
Over the past 20 years, the Ecuadorian government has awarded mangroves to fishing communities and tourism entrepreneurs. These mangrove concessions are not new in Ecuador; according to the Undersecretary for Coastal and Marine Management of the Ecuadorian Ministry of the Environment (abbreviated MAE in Spanish), 91 concessions have been granted since 2001. These concessions specify the community’s rights to use the mangrove ecosystem for artisanal fishery. In communities where the concessions have been implemented, local associations of fishermen have privileged access for fishing within the boundaries of their area (Beitl, 2015).
Within the Cayapas-Mataje Mangrove Reserve (REMACAM) the Association of Afro-Ecuadorian Women of Fishermen and Collectors of Bio-Aquatic Products of Santa Rosa Island has had for 10 years, a concession of 350 hectares assigned by the MAE since October 2018. The community members of Santa Rosa Island derive their livelihoods from bio-aquatic resources from the surrounding mangrove ecosystem, principally the bivalves of the genus Anadara, for their sustenance, also to sell them in nearby cities (Ministerio del Ambiente [MAE], 2019; USAID, 2012, p. 5). A. tuberculosa and A. similis are two bivalve species that inhabit mangroves and are found along tropical coastal regions of the Eastern Pacific, from Heroica Guaymas de Zaragoza port in Mexico to the Bay of Tumbes in Peru (Gener et al., 2009, p. 3). These species are bivalve mollusks known in Ecuador as “concha prieta,” “concha negra,” or “concha hembra” (Flores et al., 2014). These bivalves are harvested from the muddy, sandy substrate characteristic of the mangrove ecosystem during periods of low tide (Beitl, 2015).
Bivalve mollusks are benthic organisms, used as sentinel species because of their long-life cycle and sedentary lifestyle (da Silveira Fiori et al., 2018). They allow pollution levels of marine ecosystems to be evaluated, providing integrated information over time on the presence of pollutants in the environment (Boening, 1999; Erk et al., 2018). Bivalves are characterized by their ability to filter water to feed (Ruiz et al., 2018). Because of this feeding method, these animals absorb high concentrations of toxic non-essential metals such as cadmium (Cd), chromium (Cr), lead (Pb), and essential metals such as cobalt (Co), copper (Cu), manganese (Mn), and zinc (Zn), which bioaccumulate in their tissues (da Silveira Fiori et al., 2018).
In Ecuador, legal regulations related to bivalves (Ministerial Agreement N° 005 issued on August 2, 2005) regulate the minimum size (4.5 cm in length) for the extraction and commercialization of A. tuberculosa and A. similis (Ministerio de Acuacultura y Pesca [MAP], 2005). However, the regulation does not address threshold values for any contaminants, including toxic metals.
The European Commission Regulation (European Commission (EC), 2006) has established maximum levels for some contaminants, including heavy elements like Cd and Pb, of 1.0 and 1.5 mg⋅kg–1 (fresh weight), respectively, in bivalve mollusks for its consumption. The Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) have established threshold values only for Cd (2.0 mg kg–1, fresh weight) in the International Food Standards (CODEX 193) (FAO/WHO, 2019). In the case of Cr, neither the European Commission Regulation nor the FAO has established threshold values.
Regarding health risk assessment, the Environmental Protection Agency of the United States (US EPA) has established oral reference doses (RfD) of 0.001 mg kg–1 d–1 for Cd (US EPA, 2018, 2000) and 1.5 mg kg–1 d–1 for Cr (III) (US EPA-IRIS, 1998). There is no US EPA RfD value for Pb, but the United States Food and Drug Administration (FDA) interim reference level for lead corresponds to 0.003 mg d–1 for children and 0.0125 mg d–1 for adults (FDA, 2019).
Several studies on metal contamination in sediments, mangroves, and macrobenthic organisms that evaluate environmental pollution levels and health risks have been conducted in Guayas and Esmeralda provinces (Fernández-Cadena et al., 2014; Mendoza Angulo, 2014; Calle et al., 2018; Pernía et al., 2018). Nevertheless, no studies related to Pb levels have been conducted in Santa Rosa Island. For these reasons, this study aims to: a) determine the concentration of Cd, Cr, and Pb in REMACAM bivalves (A. tuberculosa and A. similis), and b) assess the health risks associated with these metals by consuming Anadara spp.
Materials and Methods
Study Area
The REMACAM is located in the northwest of Ecuador, in the province of Esmeraldas, and includes the southern part of the Tumbes-Chocó-Magdalena ecological region. Within REMACAM inhabit the community of Santa Rosa Island, comprising 310 members in 87 families. Most of the community is young (146 members between 0 and 19 years old), and the illiteracy level is 24%. The population maintains an income level below the poverty line of the national average set at 47.48 USD of monthly family income.
Santa Rosa Island community is located in the central part of REMACAM between the Pacific Ocean and Santa Rosa estuary. Four sampling sites were established based on their proximity to the ocean, and the fact that they are remote areas that could be impacted by the Santa Rosa Island population. The sites were denominated E1, on the southern border of the “Hondo” estuary, 300 m from where the Santa Rosa estuary begins. E2, on “la Tumba” beach, in the Santa Rosa estuary, 1500 m south of where the estuary begins. E3, on “al Otro Lado” beach, in the estuary that borders the north side of the settlement, and E4, “la Caleta” estuary, 500 m from the Santa Rosa estuary (Figure 1).
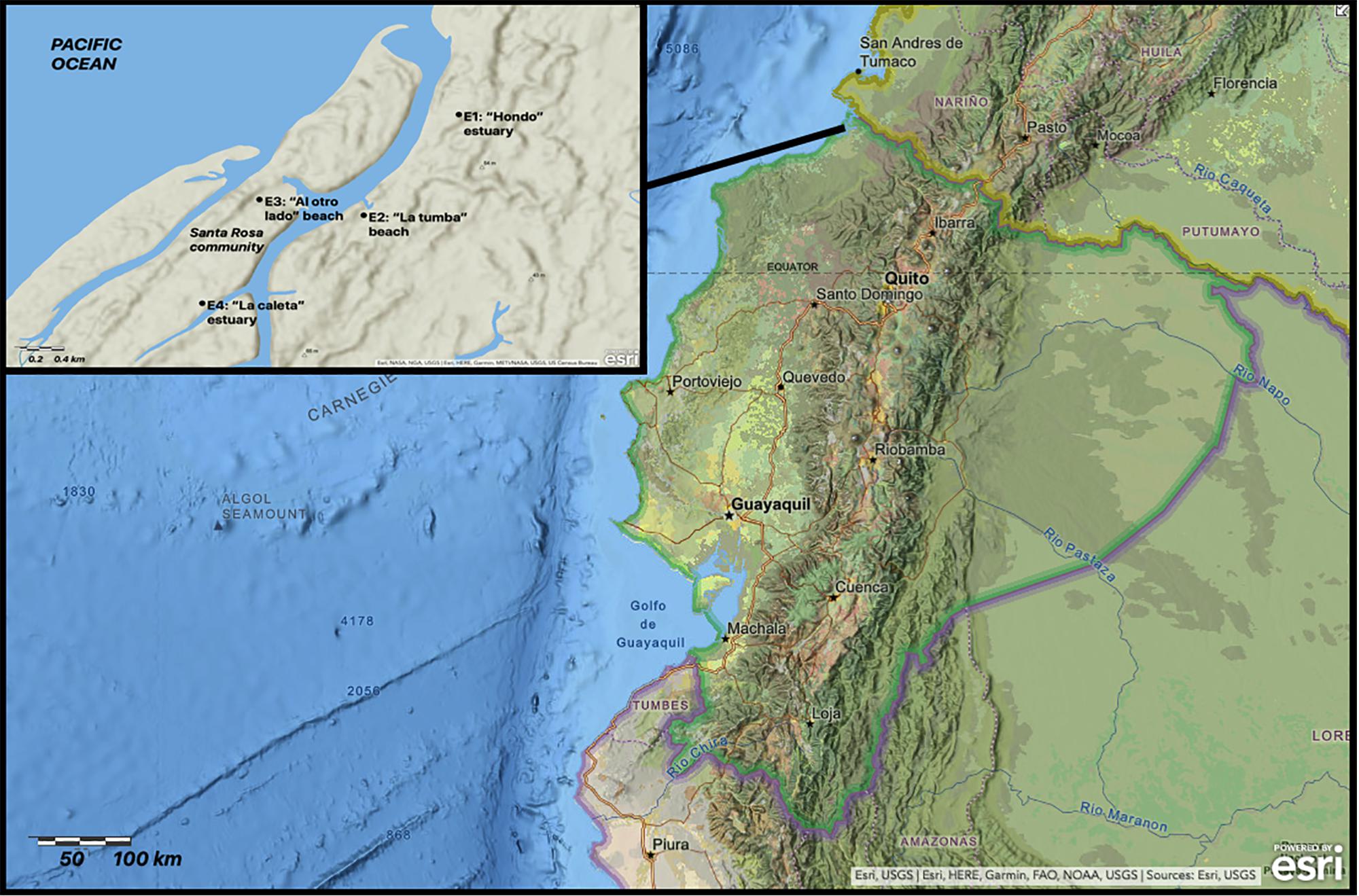
Figure 1. Location of bivalve, sediment, and leaf sampling sites (image edited from ArcGIS online and powered by esri).
Sample Collection
The samples were bought to the Santa Rosa Island community members, who carried out the sampling process using the same technique that they routinely use to collect bivalves for consumption and sale. The harvesting was done for 1 h, in which the largest possible number of bivalves were collected without considering kind or size. Four pairs of collectors were chosen for buying the samples, one for each sampling location. In addition, holes were dug to extract four surface sediment samples of about 30 cm deep using a stainless steel scoop as the SESDPROC-200-R3 reference (US EPA, 2014) and four samples of Rhizophora mangle leaves were collected, corresponding to each location.
Handling Samples Before Analysis
Fifty composite samples of the different species and sizes from the four sampling locations were formed with approximately 10 bivalves randomly selected for each sample. The samples were stored in self-sealing plastic bags, labeled, stored in a refrigerator, and transferred to the Environmental Management Laboratory of the Pontificia Universidad Católica del Ecuador in Esmeraldas (PUCE-Esmeraldas), where they were classified into 18 subgroups by species, length, and width. The soft tissues were immediately extracted to transfer to the Centro de Estudios Aplicados en Química laboratory in Quito. The sediment and leaf samples were also deposited in self-sealing plastic bags and transferred to the laboratory.
Metal Quantification
All samples including bivalves, sediment, and leaves were analyzed in triplicate, using fortifications as quality control. The lowest concentration of each metal that can be detected (detection limit) was established analytically at 0.15 mg kg–1, 0.55 mg kg–1, 0.45 mg L–1 for Cd, Cr, and Pb respectively. The minimum concentration of each metal that can be reliably determined (quantification limit) was established analytically at 0.5 mg kg–1 for Cd and 15.0 mg kg–1 for both Cr and Pb. The calibration curves were prepared in 0.01, 0.10, 0.30, 1.00 mg L–1 for Cd, and in 0.3, 1.0, 3.0, 5.0 for Cr, and Pb mg L–1, considering a correlation coefficient higher than 0.99 for its acceptation. The relative standard deviation (RSD) for each triplicate and fortification recoveries (accuracy) was calculated for each sample determination.
The refrigerated soft tissues were washed with high-quality reagent water (resistivity 18.2 MΩ⋅cm at 25°C) to eliminate sediment residue and any other kind of impurity. Water content was determined at 105°C using an oven (Memmert UM 500). Samples were dried for 72 h at 60°C in the oven until a constant weight was achieved. The dried tissues were then milled.
Approximately 0.5 g of each sample were measured in Teflon vials, and 7 mL of 65% nitric acid were added (Fisher Chemical, Certified ACS plus, CAS# 7697-37-2). Acid digestion was performed using a CEM MARS 6 microwave, applying the analytical method IPN AC-06-00 (CIIEMAD-IPN, 2011) as a reference, validated for its use for bivalve matrix. The digested samples were then filtered and made up to a final volume of 25 cm3. Metals (Cd, Cr, and Pb) were quantified with a flame atomic absorption spectrophotometer, Perkin Elmer AAnalyst 400. For blanks, samples, and calibration curve standards preparation, analytical grade reagents were employed. The triplicate analysis, fortifications of known concentrations, and certified reference material of metals in fish protein (DORM 4; National Research Council Canada) were performed as quality control. The results are presented in mg kg–1 of fresh weight.
Sediment samples were processed according to the US EPA method 3051A (US EPA, 2007). Leaves samples were processed according to the natural food method validated for its use in plant tissues (Romero-Estévez et al., 2019). Both matrixes were analyzed using a flame atomic absorption spectrophotometer.
Statistical Analyses
The arithmetic mean, standard deviation, and recovery of fortifications were calculated for all the Cd, Cr, and Pb concentrations obtained from all the samples.
The RSD values and accuracy were evaluated against the acceptable limits described in the AOAC Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals (AOAC, 2002): the RSD value acceptance limits are 11% for reproducibility, and the accuracy acceptance limits are between 80 and 115%.
In addition, for the correlation analysis between the metals content in bivalves, sediments and leaves, the correlation coefficients and p-values were calculated using an alpha (α) level of 0.05 (confidence level 95%) as an indication of statistical significance.
Calculations
Human Health Risk Assessment
In Ecuador, a traditional recipe for the bivalves included in the present study is the “ceviche,” in which approximately 20 bivalve specimens between 40 and 50 mm are used, corresponding to an approximately intake portion between 150 and 500 g (fresh weight). Eating ceviche is common for locals, who eat between 3 and 5 portions per week. For the intake calculations, the amounts of 200 g and the 400 g (fresh weight) were selected as the intake portions for children and adults respectively, considering the average number of bivalves per portion and the mean of the general meat portion.
The exposure levels (Ex) to Cd, Cr, and Pb were calculated for children (14.5 kg of body weight) and adults (70 kg of body weight) to evaluate the human health risk from the consumption of A. tuberculosa and A. similis by the community members of Santa Rosa Island. The following US EPA (2000) formula was used:
where Ex is the exposure (mg kg–1 d–1); Cx is the concentration of metal in the edible portion of samples (mg kg–1); CR is the mean amount of bivalves (kg) that are consumed daily (200 g for children and 400 g for adults), and BW is body weight (kg).
Likewise, the non-carcinogenic potential risk (Rx) for consumption was evaluated using the following equation (US EPA, 1986):
where Ex is daily exposure (mg kg–1 d–1), and RfD is the oral reference dose (mg kg–1 d–1) (US EPA-IRIS, 1998; US EPA, 2018, 2000; FDA, 2019), which corresponds to the amount of metal that could be consumed daily without any adverse health effects.
For the evaluation, Rx values lower than 1 means imperceptible risk; if Rx values are higher than 1, the exposed populations may be at risk. As the US EPA guidelines explain, “Exposure above the RfD is not recommended. The likelihood of risk is related to the degree to which exposure exceeds the RfD” (US EPA, 2000, pp. 2–53).
Individual carcinogenic risk is equivalent to the increased probability of an individual developing cancer over his/her lifetime due to exposure to the metals included in this study. The individual carcinogenic risk for Pb was estimated using the existing cancer potency provided by the US EPA (2018): 0.0085 mg⋅kg–1⋅d–1. As Cd and Cr (III) do not have slope factor values for cancer potency, individual carcinogenic risks could not be estimated. To calculate individual carcinogenic risk, the following US EPA (2001) equation was used:
Individual carcinogenic risk values lower than 10–6 were considered negligible, values between 10–6 and 10–4 were considered within an acceptable range, and values higher than 10–4 were considered intolerable (US EPA, 2001).
Besides, the allowable daily consumption rate [CRlim (g d–1)] was evaluated (US EPA, 2000) to determine the approximate amount of bivalves (g) that a person can consume per day and not present health problems, based on the RfD:
where RfD is the oral reference dose (mg⋅kg–1⋅d–1), BW is the corporal weight (kg), and Cx is each metal concentration in the samples (mg⋅kg–1).
The human health risk assessment was calculated applying different scenarios for the metal concentrations (mean, minimum, and maximum).
All the data calculations were done using Microsoft Office Professional Plus® Excel 2016.
Results and Discussion
Sample Characteristics
In this research, different soft tissue content was observed among the four sampling sites. The specimens harvested from E1 of 30 to 40 mm in length had an average meat proportion of 8.63 ± 3.65 g of meat portion and 33.7 ± 7.89% of soft tissue. From E2, specimens were bigger with an average meat portion of 13.26 ± 7.12 g and 44.4 ± 9.45% of soft tissue. From E3, specimens fell between E1 and E2 with an average of meat portion of 10.51 ± 3.93 g of and 38.2 ± 10.23% of soft tissue. Finally, from E4, specimens had 9.43 ± 4.72 g of meat portion and a 35.8 ± 12.34% of soft tissue. Samples greater than 50 mm had an average of 13.4 ± 5.31 g of meat portion and 34.7 ± 3.81% of soft tissue from E1; 20.42 ± 6.52 g of meat portion and 41.7 ± 6.87% of soft tissue from E2; 12.65 ± 6.29 g of meat portion and 25.62 ± 13.14% of soft tissue from E3; and 16.44 ± 5.68 g of meat portion and 33.96 ± 6.53% of soft tissue from E4. The mean weight of the soft tissue from A. tuberculosa and A. similis specimens between 40 and 50 mm was 10.19 ± 5.03 g, and specimens greater than 50 mm reached 14.9 ± 6.57 g.
Metals Quantification
The results of metals determination are shown in Table 1. For the Cr and Pb analysis of the bivalve samples, the results were below the quantification limits for each method. However, the approximate concentrations were estimated with the fortifications results, which were within the working range. The highest concentration of Cd was 0.560 mg kg–1 from location E2 for A. tuberculosa and 0.948 mg kg–1 from location E4 for A. similis. The highest concentration of Cr was 0.634 mg kg–1 from location E3 for A. tuberculosa and 0.730 mg kg–1 from location E4 for A. similis. Finally, for Pb, the highest concentration was 0.923 mg kg–1 for A. tuberculosa and 0.908 mg kg–1 for A. similis, both from location E3. In a study using bivalve samples from Esmeraldas Province conducted by Mendoza Angulo (2014), concentrations of 0.3 mg kg–1 for Cd and Cr were found, which corresponds to the results in the present study. No studies related to Pb levels have been conducted at Santa Rosa Island location for comparison. The Pb results for the sediment samples were lower than the quantification limit in three of the four samples; the same results were found for all leaf samples for Cr and Pb. In these cases, the concentrations were estimated using the fortification results.
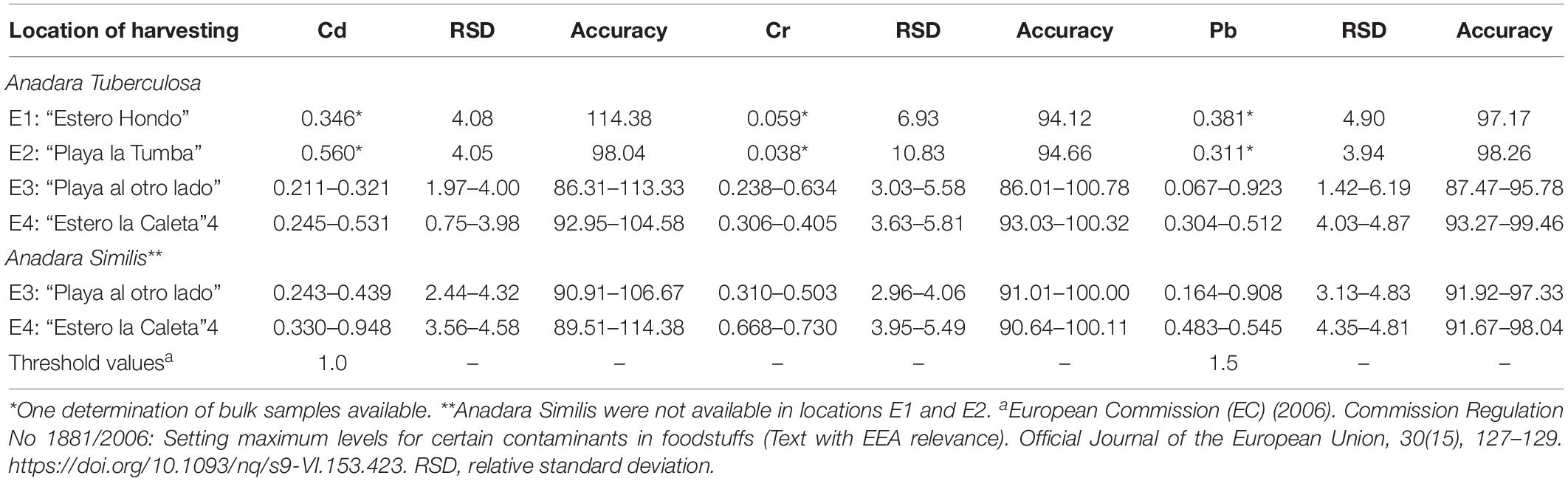
Table 1. Concentration ranges of cadmium (Cd), chromium (Cr), and lead (Pb) in A. tuberculosa and A. similis (mg kg–1), rates of the standard deviation of the triplicates (%), and accuracy (%) of the samples collected in four locations near the Isla Santa Rosa community in Esmeraldas.
In the sampling process, other species were collected and analyzed: Protothaca asperrima with a mean concentration of 0.15, 0.41, and 0.49 mg kg–1 for Cd, Cr, and Pb, respectively, and Polymesoda inflata with a mean concentration of 0.27, 0.74, and 0.36 mg kg–1 for Cd, Cr, and Pb, respectively. In all the cases, the highest RSD were 5.43, 5.19, and 5.56% for Cd, Cr, and Pb, respectively, and the fortification recovery rates (accuracy) were between 81.82 and 101.82%. These species are not commonly used for consumption, thus the risk assessment related was no calculated. The metal content was in the same order as the content obtained for the Anadara spp. samples.
The certified reference material of fish protein recovery results were 84.91% for Cd, 86.19% for Cr, and 91.59% for Pb.
For the sediment results, the mean values of the metal concentrations were 2.14, 29.99, and 12.37 mg kg–1 for Cd, Cr, and Pb, respectively. The RSD values were under 5.53, 2.65, and 2.93% for Cd, Cr, and Pb, respectively, and the recoveries were between 80.07 and 104.58%. The highest value corresponded to location E3.
For Cd, Cr, and Pb content, the results are varied, and no relation could be found between the bivalves and sediments metal content. For A. tuberculosa the correlation coefficients were 0.2186 (p = 3.04E-06), 0.0887 (p = 5.46E-06), and 0.1044 (p = 6.75E-06), for Cd, Cr, and Pb, respectively. In the case of A. similis the correlation coefficients were 0.3356 (p = 0.0019), 0.8393 (p = 0.0009), and 0.0105 (p = 0.0011) for Cd, Cr, and Pb, respectively. For all cases, the p-values were lower than α = 0.05.
The results of the present study are in agreement with some authors who concluded that there is no correlation between the metal content between sediment and bivalve species (Chu et al., 1990; Amiard et al., 2007; Lias et al., 2013). This difference may be related to sediment properties such as acid-volatile sulfides, organic matter, texture, geology, organism behaviors among others those influencing the bioavailability of metals (Zhang et al., 2014). On the other hand, the presence of metals in the sediments is related to other parameters as pH, salinity, conductivity, and temperature. The pH contributes to the efficiency of the interaction between the metal ion and the adsorbent surface (Saranya et al., 2018); water salinity is another important factor because the Cl– ion can form various complexes with metals, allowing the exchange, increase or decrease of metals in sediment-aquatic environment (Harter and Naidu, 2001); the electrical conductivity is proportional is directly related to cations or anions content including metals; the temperature has a direct influence on the solubility of heavy metals.
The mean concentrations from the Rhizophora mangle leaves were 2.21, 4.38, and 3.29 mg⋅kg–1 for Cd, Cr, and Pb, respectively. The RSD values were under 5.88, 5.83, and 5.00% for Cd, Cr, and Pb, respectively, and the recoveries were between 80.89 and 106.10%. In this case, the highest values corresponded to locations E1, E2, and E3 for Pb, Cr, and Cd, respectively, and did not show a relation to sediment or bivalve results.
In the comparison between the metal content of leaves and bivalve samples, there was not a correlation between the results of the three metals. The correlation coefficients were 0.0731 (p = 0.0025), 0.0722 (p = 0.0004), and 0.0778 (p = 4.57E-07), for Cd, Cr, and Pb, respectively for A. tuberculosa. In the case of A. similis the correlation coefficients were 0.3356 (p = 0.0480), 0.8393 (p = 0.0108), and 0.0105 (p = 5.05E-05) for Cd, Cr, and Pb, respectively. For all cases, the p values were lower than α = 0.05. Nevertheless, Rhizophora mangle leaves can be used as important bioindicators of metal contamination in mangrove ecosystems (Pinheiro et al., 2012).
Location E3 is nearest to the community of Santa Rosa Island, and in this location, there is little water flow, in a curved area where sediment can accumulate. Location E4 is farther away from the community and belongs to the “La Caleta” estuary riverbed. The trace metal contamination is assumed to be from the natural composition of soils and erosion (Soborino-Figueroa et al., 2007; Aouini et al., 2018; Arumugam et al., 2018; Fasano et al., 2018), There is also illegal mining in localities upstream of river beds that flow into the area (Global Initiative against Transnational Organized Crime, 2016). Also, the inadequate management of household waste, incorrect final disposal of fuels for the repair of boats and outboard motors, pollutants that are dumped directly into the mangrove (Echeverría, 2019).
Ecuadorian laws do not have specific regulations related to metal concentrations in bivalves; nevertheless, when applying the European Commission and FAO regulations, the results of the present study are lower than the Cd and Pb threshold values.
Regarding mangrove sediments, Ecuadorian regulations do not establish specific acceptance limits for contaminants; however, Ministerial Agreement N° 097 established regulation for soil quality in general, and the maximum acceptable limits for Cd, Cr, and Pb are 0.5, 54, and 19 mg⋅kg–1, respectively (Ministerio del Ambiente [MAE], 2015). The Canadian interim sediment quality guidelines (ISQGs) also set maximum limits for these metals: 0.7 mg⋅kg–1 for Cd (Canadian Council of Ministers of the Environment [CCME], 1999), 52.3 mg⋅kg–1 for Cr (Canadian Council of Ministers of the Environment [CCME], 1995), and 30.2 mg⋅kg–1 for Pb (Canadian Council of Ministers of the Environment [CCME], 1999). The concentrations obtained in the present study were 2.14, 29.99, and 12.37 mg⋅kg–1 for Cd, Cr, and Pb, respectively. Thus, the concentrations found were lower than the Ecuadorian and Canadian regulations for both Cr and Pb. In the case of Cd, the average concentration was approximately four times the limit established in Ecuadorian regulations and three times the limit established in Canadian regulations, which may be due to the presence of cadmium in water and soils (Fernández-Cadena et al., 2014; Echeverría, 2019).
Human Health Risk Assessment
As Acosta and Lodeiros (2004) mention, the consumption of mollusks, like bivalves, contributes to a potentially toxic metal intake. Toxic metals can elevate the risk of chronic poisoning for people living in coastal areas, and also to mangroves (Acosta and Lodeiros, 2004; Fernández-Cadena et al., 2014; Aguirre-Rubí et al., 2018a, b; Loaiza et al., 2018; Pernía et al., 2018).
For the Ex results, all Cd levels for A. tuberculosa and A. similis exceeded the respective RfD value (0.001 mg⋅kg–1⋅d–1). Exposure to Cd is mainly associated with increased urinary excretion of low molecular weight proteins, with repercussions on kidney dysfunction and bone disease (Itai Itai disease) in exposed populations, and also with the development of cancer (Godt et al., 2006).
In Cr, all results were quite lower than the RfD value (1.5 mg⋅kg–1⋅d–1), and for Pb, the results were lower than the RfD values (0.003 mg⋅d–1 for children and 0.0125 mg⋅d–1 for adults), except in children for the mean and maximum Pb concentration scenarios for both bivalve species. Moreover, the results show that in all cases (children and adults for A. similis and A. tuberculosa), Cd potential risk levels are higher than those for Cr and Pb; therefore, more Cd toxicity exists per amount of bivalves ingested (Tables 2 and 3). The individual carcinogenic risk showed values lower than 10–4 for A. tuberculosa and A. similis, which is considered within an acceptable range. The higher value was in children for the maximum Pb concentration scenario in A. tuberculosa. Exposure to elevated levels of Pb has serious consequences on the health of children, attacks the brain and central nervous system. When there is contamination at lower levels, there are no obvious symptoms, however, it can affect the brain development of children, reduced IQ, changes in behavior, attention disorders, antisocial behavior. Furthermore, exposure to lead also causes anemia, hypertension, kidney failure, immunotoxicity, and toxicity to the reproductive organs, irreversible neurological, and behavioral effects (WHO, 2019). Counter et al. (2015) have found that the blood Pb levels observed in Ecuadorian infants (33.6 ± 28.9 μg dl–1) and young children (27.9 ± 22.5 μg dl–1) are higher than the World Health Organization level of concern (10 μg dl–1), and Centers for Disease Control and Prevention current reference value (5 μg dl–1). This worrying situation means that pediatric Pb intoxication persists in developing countries where Pb contamination exists.
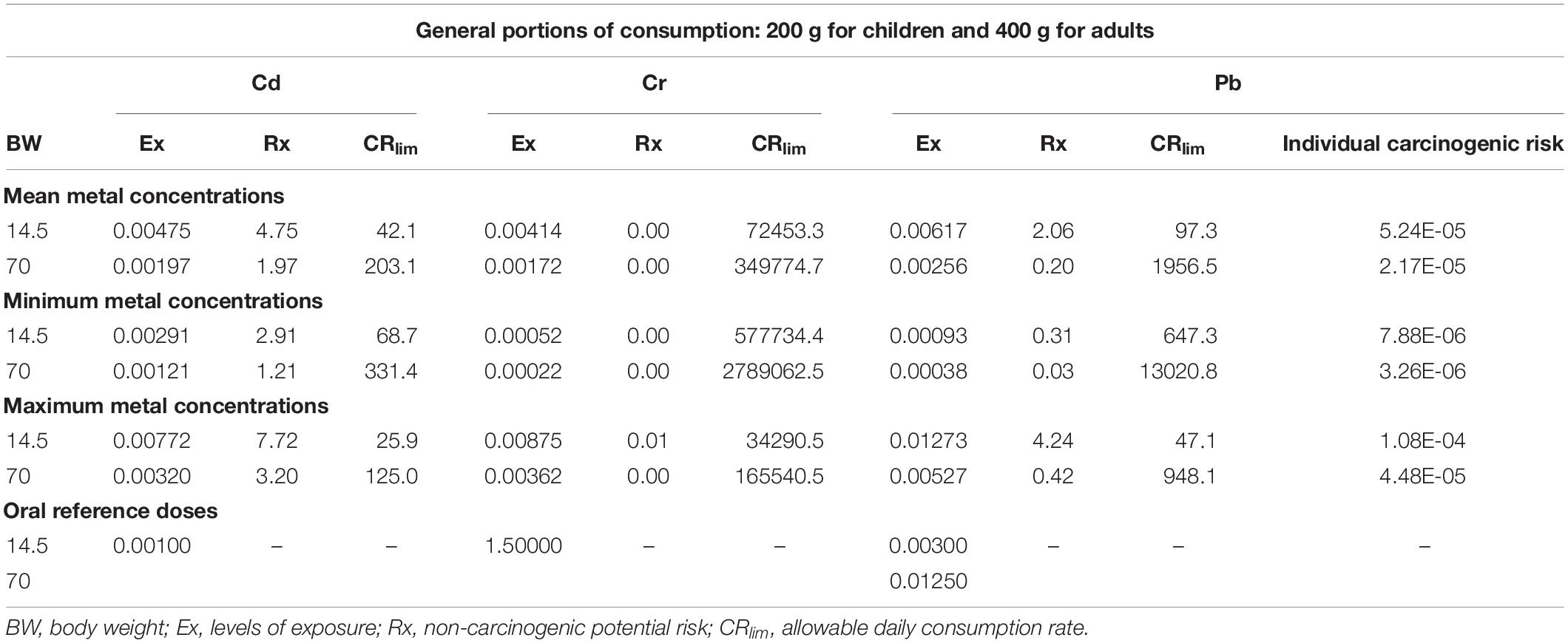
Table 2. Anadara tuberculosa exposure levels (mg kg–1 d–1), non-carcinogenic potential risk (no units), individual carcinogenic risk for Pb (no units), and the allowable daily consumption rate (g d–1) for Cd, Cr, and Pb, considering the mean, minimum, and maximum metal concentrations for child and adult mean body weights (kg).
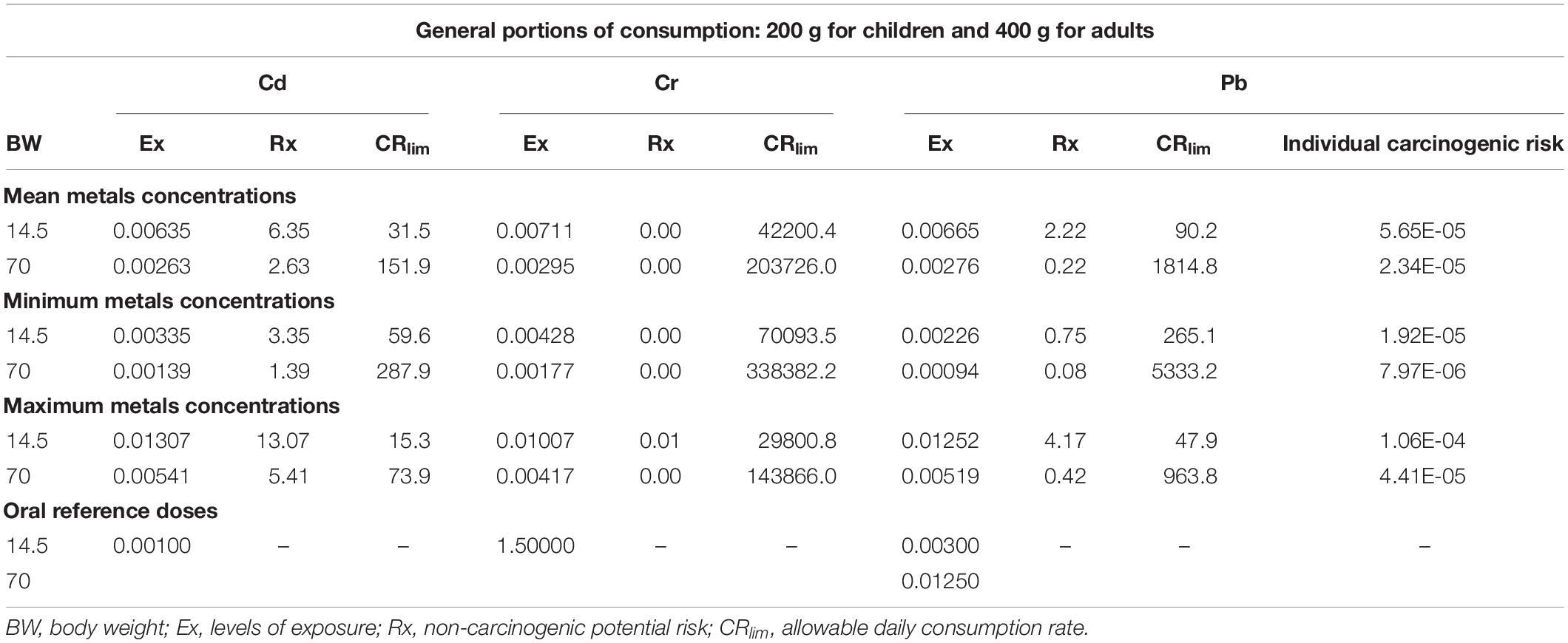
Table 3. Anadara Similis exposure levels (mg kg–1 d–1), non-carcinogenic potential risk (no units), individual carcinogenic risk for Pb (no units), and the allowable daily consumption rate (g d–1) for Cd, Cr, and Pb, considering the mean, minimum, and maximum metal concentrations for child and adult mean body weights (kg).
Finally, the allowable daily consumption rate (CRlim) in grams of A. tuberculosa was estimated considering that Cd poses the highest potential risk. For the mean, minimum, and maximum Cd concentration scenarios, child CRlim values were 42.1, 68.7, and 25.9 g, respectively, and adult values were 203.1, 331.4, and 125.0 g, respectively. In the case of A. similis (Table 3), for each scenario, the child CRlim values were 31.5, 59.6, and 15.3 g of bivalves; the adult CRlim values were 151.9, 287.9, and 73.9 g of bivalves. The higher results indicate that more consumption of bivalves than those obtained in the calculations could imply a high risk to any health for both children and adults. The FDA recommendations cited by Soborino-Figueroa et al. (2007) suggest the consumption of mollusks from 28 to 83 g as a safe portion. In the case of this study, the recommended values are higher than those recommended by Soborino-Figueroa et al. (2007).
Conclusion
There is heterogeneity in the content of Cd, Cr, and Pb found in the bivalve samples of the present study. Additionally, when comparing the results of the content of metals in the sediments and the leaves collected in the same locations of bivalves, no correlation was found. These may be an indication that the presence of metals in mangrove substrates (sediments) is not the only aspect to be considered to explain a high intake by natural species as bivalves (Anadara spp.), and vegetation (Rhizophora mangle). In addition, these sediments results can be used as a baseline that indicates no metal enrichment of any sort in the locations areas. On the other hand, the present study is aimed to be the first approach to determine a possible contamination of Cd, Cr, and Pb, in the area inside the REMACAM natural reserve. The results obtained suggest that more studies should be done to a wider evaluation of metals contamination including other locations within the REMACAM area.
As seen in the health risk assessment results for children (portion corresponding to 200 g) and adults (portion corresponding to 400 g), it is evident that consumption of a bivalve portion contains concentrations of Cd exceeding the RfD values from US EPA (2018, 2000). In the case of Pb, the concentration values for children (mean and maximum scenarios) were above de FDA (2019) values. In all cases, the consumption of bivalves could imply a health risk to the community members of Santa Rosa Island.
Many communities that derive their livelihoods from bio-aquatic resources are isolated; this fact is a limiting factor for the periodic monitoring and hinders the implementation of treatment systems as depuration for the elimination of Cd, Cr, and Pb. Furthermore, economic limitations make development activities depend on external resources, which, in most cases, are difficult to achieve.
In Santa Rosa Island, the sources of trace metal contamination cannot be completely determined because they are both natural and anthropogenic. This first approach aimed to have a general evaluation about the metals presence and the community risk exposure, but further investigations are needed to find out the principal source of contamination.
It is necessary to continue the studies related to toxic metals content in bio-aquatic resources and its relationship with public safety associated with the consumption of food that contains toxic metals. It also imperative to keep monitoring the locations where it is presumed that metal contamination exists.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
DR-E and MD: conceptualization. DR-E, HN, and MD: visualization. MD and HN: resources. MD: project administration and funding acquisition. DR-E: methodology and validation. DR-E, KS-F, ER, and GD: formal analysis and investigation. DR-E and GY-J: data curation, writing – original draft, review, and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank to the Pontificia Universidad Católica del Ecuador Quito, Esmeraldas and Manabí headquarters for the support for the project “Ciudad Abierta.”
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2020.00134/full#supplementary-material
References
Acosta, V., and Lodeiros, C. (2004). Heavy metals in the clam Tivela mactroides Born, 1778 (Bivalvia: Veneridae) from coastal localities with different degrees of contamination in Venezuela. Ciencias Mar. 30, 323–333. doi: 10.7773/cm.v30i2.183
Aguirre-Rubí, J., Luna-Acosta, A., Etxebarría, N., Soto, M., Espinoza, F., Ahrens, M. J., et al. (2018a). Chemical contamination assessment in mangrove-lined Caribbean coastal systems using the oyster Crassostrea rhizophorae as biomonitor species. Environ. Sci. Pollut. Res. 25, 13396–13415. doi: 10.1007/s11356-017-9159-2
Aguirre-Rubí, J., Luna-Acosta, A., Ortiz-Zarragoitia, M., Zaldibar, B., Izagirre, U., Ahrens, M. J., et al. (2018b). Assessment of ecosystem health disturbance in mangrove-lined Caribbean coastal systems using the oyster Crassostrea rhizophorae as sentinel species. Sci. Total Environ. 618, 718–735. doi: 10.1016/j.scitotenv.2017.08.098
Amiard, J.-C., Geffard, A., Amiard-Triquet, C., and Crouzet, C. (2007). Relationship between the lability of sediment-bound metals (Cd, Cu, Zn) and their bioaccumulation in benthic invertebrates. Estuar. Coast. Shelf Sci. 72, 511–521. doi: 10.1016/j.ecss.2006.11.017
AOAC (2002). Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals. AOAC Int. 1–38. Available online at: http://members.aoac.org/aoac_prod_imis/AOAC_Docs/StandardsDevelopment/SLV_Guidelines_Dietary_Supplements.pdf (accessed May 15, 2020).
Aouini, F., Trombini, C., Volland, M., Elcafsi, M., and Blasco, J. (2018). Assessing lead toxicity in the clam Ruditapes philippinarum: bioaccumulation and biochemical responses. Ecotoxicol. Environ. Saf. 158, 193–203. doi: 10.1016/j.ecoenv.2018.04.033
Arumugam, G., Rajendran, R., Shanmugam, V., Sethu, R., and Krishnamurthi, M. (2018). Flow of toxic metals in food-web components of tropical mangrove ecosystem, Southern India. Hum. Ecol. Risk Assess. 24, 1367–1387. doi: 10.1080/10807039.2017.1412819
Beitl, C. M. (2015). Mobility in the mangroves: catch rates, daily decisions, and dynamics of artisanal fishing in a coastal commons. Appl. Geogr. 59, 98–106. doi: 10.1016/j.apgeog.2014.12.008
Boening, D. W. (1999). An evaluation of bivalves as biomonitors of heavy metals pollution in marine waters. Environ. Monit. Assess. 55, 459–470. doi: 10.1023/A:1005995217901
Calle, P., Monserrate, L., Medina, F., Calle, D. M., Tirapé, A., Montiel, M., et al. (2018). Mercury assessment, macrobenthos diversity and environmental quality conditions in the Salado Estuary (Gulf of Guayaquil, Ecuador) impacted by anthropogenic influences. Mar. Pollut. Bull. 136, 365–373. doi: 10.1016/j.marpolbul.2018.09.018
Canadian Council of Ministers of the Environment [CCME] (1995). Canadian Sediment Quality Guidelines for the Protection of Aquatic Life: Chromium. Can. Counc. Minist. Environ. EPC-98E 35. Available online at: http://ceqg-rcqe.ccme.ca/download/en/233 (accessed May 15, 2020).
Canadian Council of Ministers of the Environment [CCME] (1999). Canadian Sediment Quality Guidelines for the Protection of Aquatic Life: Cadmium. Can. Environ. Qual. Guidel. 5. Available online at: http://ceqg-rcqe.ccme.ca/download/en/231 (accessed May 15, 2020)
Chu, K. H., Cheung, W. M., and Lau, S. K. (1990). Trace metals in bivalves and sediments from Tolo Harbour, Hong Kong. Environ. Int. 16, 31–36. doi: 10.1016/0160-4120(90)90202-h
CIIEMAD-IPN (2011). Método General por Microondas de Digestión Ácida en Matrices Ambientales 0, 1–15. Available online at: https://www.ciiemad.ipn.mx/assets/files/ciiemad/docs/sgc/procedimientos/IPN_AC-06-00.pdf (accessed May 15, 2020)
Counter, S. A., Buchanan, L. H., and Ortega, F. (2015). Blood lead levels in Andean infants and young children in Ecuador: an international comparison. J. Toxicol. Environ. Part A 78, 778–787. doi: 10.1080/15287394.2015.1031050
da Silveira Fiori, C., de Castro Rodrigues, A. P., Castro Vieria, T., Sabadini-Santos, E., and Dausacker Bidone, E. (2018). An alternative approach to bioaccumulation assessment of methyl-Hg, total- Hg, Cd, Pb, Zn in bivalve Anomalocardia brasiliana from Rio de Janeiro bays. Mar. Pollut. Bull. 135, 418–426. doi: 10.1016/j.marpolbul.2018.07.045
Echeverría, K. M. (2019). Metales Pesados en Agua, Sedimentos y Raíces de Rhizophora mangle de la Reserva Ecológica Manglares Cayamas Mataje, Provincia de Esmeraldas. Ph. D. Thesis, Pontificia Universidad Católica del Ecuador sede Ibarra, Ecuador.
Erk, M., Ivankoviæ, D., Župan, I., Èulin, J., Dragun, Z., Puljas, S., et al. (2018). Changes in the tissue concentrations of trace elements during the reproductive cycle of Noah’s Ark shells (Arca noae Linnaeus, 1758). Mar. Pollut. Bull. 133, 357–366. doi: 10.1016/j.marpolbul.2018.05.054
Esposito, G., Meloni, D., Abete, M. C., Colombero, G., Mantia, M., Pastorino, P., et al. (2018). The bivalve Ruditapes decussatus: A biomonitor of trace elements pollution in Sardinian coastal lagoons (Italy). Environ. Pollut. 242(Pt B), 1720–1728. doi: 10.1016/j.envpol.2018.07.098
European Commission (EC) (2006). Commission regulation No 1881/2006: setting maximum levels for certain contaminants in foodstuffs (Text with EEA relevance). Off. J. Eur. Union. 30, 127–129.
FAO/WHO (2019). General Standard for Contaminants and Toxins in Food and Feed CXS 193-1995. Available online at: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B193-1995%252FCXS_193e.pdf (Accessed May 15, 2020).
Fasano, E., Arnese, A., Esposito, F., Albano, L., Masucci, A., Capelli, C., et al. (2018). Evaluation of the impact of anthropogenic activities on arsenic, cadmium, chromium, mercury, lead, and polycyclic aromatic hydrocarbon levels in seafood from the Gulf of Naples, Italy. J. Environ. Sci. Heal. Part A 53, 786–792. doi: 10.1080/10934529.2018.1445075
FDA (2019). Lead in Food, Foodwares, and Dietary Supplements. Available online at: https://www.fda.gov/food/metals-and-your-food/lead-food-foodwares-and-dietary-supplements (Accessed May 15, 2020).
Fernández-Cadena, J. C., Andrade, S., Silva-Coello, C. L., and De la Iglesia, R. (2014). Heavy metal concentration in mangrove surface sediments from the north-west coast of South America. Mar. Pollut. Bull. 82, 221–226. doi: 10.1016/j.marpolbul.2014.03.016
Flores, L., Licandeo, R., Cubillos, L. A., and Mora, E. (2014). Intra-specific variability in life-history traits of Anadara tuberculosa (Mollusca: Bivalvia) in the mangrove ecosystem of the Southern coast of Ecuador. Rev. Biol. Trop. 62, 473–482.
Gener, R. L., Rivas, F., and Argüello, G. (2009). Estudio de Mercado de la Concha negra (Anadara similis y Anadara tuberculosa) en Nicaragua: Comercialización con Garantía de Inocuidad. Ph. D. Thesis, Universidad Centroamericana, Managua.
Global Initiative against Transnational Organized Crime (2016). Organized Crime and Illegally Mined Gold in Latin America. Ginebra, Suiza: Global Initiative. Available online at: https://arcominero.infoamazonia.org/GIATOC-OC_Illegally-Mined-Gold-in-Latin-America-3c3f978eef80083bdd8780d7c5a21f1e.pdf (accessed May 15, 2020).
Godt, J., Scheidig, F., Grosse-Siestrup, C., Esche, V., Brandenburg, P., Reich, A., et al. (2006). The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 1:22. doi: 10.1186/1745-6673-1-22
Harter, R., and Naidu, R. (2001). An assessment of environmental and solution parameter impact on trace-metal sorption by soils. Soil. Sci. Soc. Am. J. 65:597. doi: 10.2136/sssaj2001.653597x
Jiang, Q., He, J., Ye, G., and Christakos, G. (2018). Heavy metal contamination assessment of surface sediments of the East Zhejiang coastal area during 2012 – 2015. Ecotoxicol. Environ. Saf. 163, 444–455. doi: 10.1016/j.ecoenv.2018.07.107
Lias, K., Jamil, T., and Nor Aliaa, S. A. (2013). A preliminary study on heavy metal concentration in the marine bivalves marcia marmorata species and sediments collected from the coastal area of Kuala Perlis, North Of Malaysia. IOSR-JAC. 4, 48–54. doi: 10.9790/5736-0414854
Loaiza, I., De Troch, M., and De Boeck, G. (2018). Potential health risks via consumption of six edible shellfish species collected from Piura – Peru. Ecotoxicol. Environ. Saf. 159, 249–260. doi: 10.1016/j.ecoenv.2018.05.005
Mendoza Angulo, H. (2014). Niveles de Acumulación de Metales Pesados y Contaminantes Orgánicos en Moluscos Bivalvos del Género Anadara y su Vinculación con Actividades Económicas en la Provincia de Esmeraldas como base para una Propuesta de Regulación de Límites Máximos Permisibles. Ph. D. Thesis, Pontificia Universidad Católica del Ecuador sede Esmeraldas, Ecuador.
Ministerio de Acuacultura y Pesca [MAP] (2005). Acuerdo Ministerial No 005. Available online at: http://acuaculturaypesca.gob.ec/subpesca93-acuerdo-ministerial-n-005-concha.html (accessed May 15, 2020).
Ministerio del Ambiente [MAE] (2015). Acuerdo Ministerial 097: Norma de Calidad Ambieltal del Recurso Suelo y Criterios de Remediación para Suelos Contaminados. Avaialable online at: http://faolex.fao.org/docs/pdf/ecu112181.pdf (Accessed May 15, 2020).
Ministerio del Ambiente [MAE] (2019). Plan de Manejo para el Uso Sostenible y Custodia de Manglar para la Asociación de Mujeres Afroecuatorianas de Pescadoras y Recolectoras de Productos Bioacuáticos 18 de octubre del Manglar de Santa Rosa. Esmeraldas: Ministerio del Ambiente de Ecuador.
Pernía, B., Mero, M., Cornejo, X., Ramírez, N., and Ramírez, L. (2018). Determinación de cadmio y plomo en agua, sedimento y organismos bioindicadores en el Estero Salado, Ecuador (Determination of cadmium and lead in water, sediment and bioindicator organisms in Estero Salado, Ecuador). Enfoque UTE 9, 89–105. doi: 10.29019/enfoqueute.v9n2.246
Pinheiro, M. A. A., Silva, P. P. G. E., Duarte, L. F., de, A., Almeida, A. A., and Zanotto, F. P. (2012). Accumulation of six metals in the mangrove crab Ucides cordatus (Crustacea: Ucididae) and its food source, the red mangrove Rhizophora mangle (Angiosperma: Rhizophoraceae). Ecotoxicol. Environ. Saf. 81, 114–121. doi: 10.1016/j.ecoenv.2012.05.004
Romero-Estévez, D., Yánez-Jácome, G. S., Simbaña-Farinango, K., Vélez-Terreros, P. Y., and Navarrete, H. (2019). Evaluation of two sample preparation methods for the determination of cadmium, nickel and lead in natural foods by Graphite Furnace Atomic Absorption Spectrophotometry. Uni. Sci. 24, 497–521. doi: 10.11144/Javeriana.SC24-3.eots
Ruiz, M. D., Iriel, A., Yusseppone, M. S., Ortiz, N., Di Salvatore, P., Fernández Cirelli, A., et al. (2018). Trace metals and oxidative status in soft tissues of caged mussels (Aulacomya atra) on the North Patagonian coastline. Ecotoxicol. Environ. Saf. 155, 152–161. doi: 10.1016/j.ecoenv.2018.02.064
Saranya, J., Parthasarathi, C., Darwin, R., Kartheek, C., and Sucharita, C. (2018). Effect of pH on transport and transformation of Cu-sediment complexes in mangrove systems. Mar. Pollut. Bull. 133, 920–929. doi: 10.1016/j.marpolbul.2018.03.054
Soborino-Figueroa, A., Cáceres-Martínez, C., and Rosas-Cedillo, R. (2007). Evaluación del riesgo por consumir moluscos contaminados con cadmio, cromo y plomo. Rev. Hidrobiológica. 17, 49–58.
Spalding, M., Kainuma, M., and Collins, L. (2010). World Atlas of Mangroves. A collaborative project of ITTO, ISME, FAO, UNEP-WCMC, UNESCO-MAB, UNU-INWEH and TNC. London (UK): Earthscan, London. 319 pp. Data layer from the World Atlas of Mangroves. In Supplement to: Spalding et al. Cambridge (UK): UNEP World Conservation Monitoring Centre. Available online at: http://data.unep-wcmc.org/datasets/22 (Accessed May 15, 2020).
US EPA (1986). Guidelines for the health risk assessment of chemical mixtures. Risk Assess. Forum 51, 34014–34025.
US EPA (2000). Guidance for assessing chemical contaminant data for use in fish advisories. Risk Assess. Fish Consump. Limits 3:34.
US EPA (2001). Risk Assessment Guidance for Superfund: Part A, Process for Conducting Probabilistic Risk Assessment 3. Available online at: https://www.epa.gov/sites/production/files/2015-09/documents/rags3adt_complete.pdf (accessed May 15, 2020).
US EPA (2007). Method 3051A. Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils. Available online at: https://www.epa.gov/esam/us-epa-method-3051a-microwave-assisted-acid-digestion-sediments-sludges-and-oils (accessed May 15, 2020).
US EPA (2014). Sediment Sampling. SESDPROC-200-R4. Available online at: https://www.epa.gov/sites/production/files/2015-06/documents/Sediment-Sampling.pdf (Accessed May 15, 2020).
US EPA (2018). Regional Screening (RBL) Summary Table. Available online at: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed May 15, 2020).
US EPA-IRIS (1998). Chromium (III), insoluble salts; CASRN 16065-83-1. Integr. Risk Inf. Syst. U.S. Chem. Assess. Summ. Natl. Cent. Environ. Assess. Available online at: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0028_summary.pdf (accessed May 15, 2020).
USAID (2012). Co-manejo de Bivalvo “Concha Negra” (Anadara ssp) en Aserradores, Nicaragua. Programa Reg. USAID para el manejo Recur. acuáticos y Altern. económicas. Available online at: http://repositorio.concytec.gob.pe/bitstream/20.500.12390/200/3/2016_Pretell_Identificacion-bacterias-cultivables.pdf (accessed May 15, 2020).
WHO (2019). Lead poisoning and health. Available online at: https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health (accessed May 15, 2020).
Keywords: Anadara tuberculosa, Anadara similis, human health, metal intake, toxicology
Citation: Romero-Estévez D, Yánez-Jácome GS, Dazzini Langdon M, Simbaña-Farinango K, Rebolledo Monsalve E, Durán Cobo G and Navarrete H (2020) An Overview of Cadmium, Chromium, and Lead Content in Bivalves Consumed by the Community of Santa Rosa Island (Ecuador) and Its Health Risk Assessment. Front. Environ. Sci. 8:134. doi: 10.3389/fenvs.2020.00134
Received: 22 May 2020; Accepted: 16 July 2020;
Published: 06 August 2020.
Edited by:
Bernardo Duarte, Center for Marine and Environmental Sciences (MARE), PortugalReviewed by:
Mário Sousa Diniz, Faculty of Sciences and Technology, New University of Lisbon, PortugalMichael Edward Deary, Northumbria University, United Kingdom
Copyright © 2020 Romero-Estévez, Yánez-Jácome, Dazzini Langdon, Simbaña-Farinango, Rebolledo Monsalve, Durán Cobo and Navarrete. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Romero-Estévez, dfromero@puce.edu.ec; Mónica Dazzini Langdon, mdazzini435@puce.edu.ec
 David Romero-Estévez
David Romero-Estévez Gabriela S. Yánez-Jácome
Gabriela S. Yánez-Jácome Mónica Dazzini Langdon
Mónica Dazzini Langdon Karina Simbaña-Farinango
Karina Simbaña-Farinango Eduardo Rebolledo Monsalve
Eduardo Rebolledo Monsalve Gabriel Durán Cobo
Gabriel Durán Cobo Hugo Navarrete1
Hugo Navarrete1