Insight Into the Role of Ferroptosis in Non-neoplastic Neurological Diseases
- Department of Neurosurgery, The Second Affiliated Hospital of Nanchang University, Nanchang, China
Ferroptosis is an iron-dependent form of cell death characterized by the accumulation of intracellular lipid reactive oxygen species (ROS). Ferroptosis is significantly different from other types of cell death including apoptosis, autophagy, and necrosis, both in morphology and biochemical characteristics. The mechanisms that are associated with ferroptosis include iron metabolism, lipid oxidation, and other pathophysiological changes. Ferroptosis inducers or inhibitors can influence its occurrence through different pathways. Ferroptosis was initially discovered in tumors, though recent studies have confirmed that it is also closely related to a variety of neurological diseases including neurodegenerative disease [Alzheimer’s disease (AD), Parkinson’s disease (PD), etc.] and stroke. This article reviews the definition and characteristics of ferroptosis, the potential mechanisms associated with its development, inducers/inhibitors, and its role in non-neoplastic neurological diseases. We hope to provide a theoretical basis and novel treatment strategies for the treatment of central nervous system diseases by targeting ferroptosis.
Introduction
Ferroptosis, an iron-dependent type of programmed cell death, is different from apoptosis, autophagy, and necrosis with regards to both morphology and biochemistry (Stockwell et al., 2017). Previous studies have shown that ferroptosis is not only related to tumorigenesis (Liang et al., 2019) but also involved in the processes of various neurological diseases (Alim et al., 2019; Derry et al., 2020). However, its specific role and mechanism in tumorigenesis and neurological diseases are still unclear. This article reviews the mechanism of ferroptosis and its role in non-neoplastic neurological diseases to provide novel ideas for the development of therapeutic drugs that target ferroptosis.
Definition and Characteristics of Ferroptosis
Dolma et al. (2003) found that the compound erastin can selectively kill RAS-mutated tumor cells, without any subsequent changes in nuclear morphology, DNA fragmentation, and other processes. Interestingly, erastin cannot be repressed by caspase inhibitors. Later, other scholars found that this ferroptosis can be inhibited by iron-chelating agents, and is accompanied by an increase in lipid reactive oxygen species (ROS) in the cell cytoplasm (Yang and Stockwell, 2008). Therefore, Dixon et al. (2012) named this form of cell death ferroptosis and defined it as an iron-dependent, non-apoptotic form of cell death characterized by intracellular lipid ROS accumulation, after which the concept was widely accepted by other researchers (Cao and Dixon, 2016; Xie et al., 2016).
Apoptosis, which refers to a process of programmed cell death dependent on caspase-3, mainly manifests as cell shrinkage, the disappearance of the mitochondrial membrane potential, chromatin concentration, and plasma membrane invagination to form apoptotic bodies (Williams and Smith, 1993). Autophagy is a process in which cells use lysosomes to degrade damaged organelles or macromolecules, The autophagy process is accompanied by the expansion of the Golgi apparatus and endoplasmic reticulum, nuclear condensation, and formation of the autophagosome (Mizushima and Komatsu, 2011). Necrosis refers to cell death that is caused by physical, chemical, or biological factors. It is characterized by swelling of the organelles, rupturing of the plasma membrane, and the release of cellular contents, all of which lead to the development of inflammation (Wallach et al., 2016). On the other hand, ferroptosis is characterized by a complete plasma membrane, increased mitochondrial membrane density, a reduced or disappeared crest, increased release of oxidized polyunsaturated fatty acids (PUFAs), and lipid ROS in the cytoplasm, all of which can be inhibited by iron-chelating agents (Stockwell et al., 2017).
Mechanism of Ferroptosis
Iron metabolism dysfunction, intracellular lipid ROS production, and degradation imbalance contribute to the occurrence of ferroptosis.
Iron Metabolism and Ferroptosis
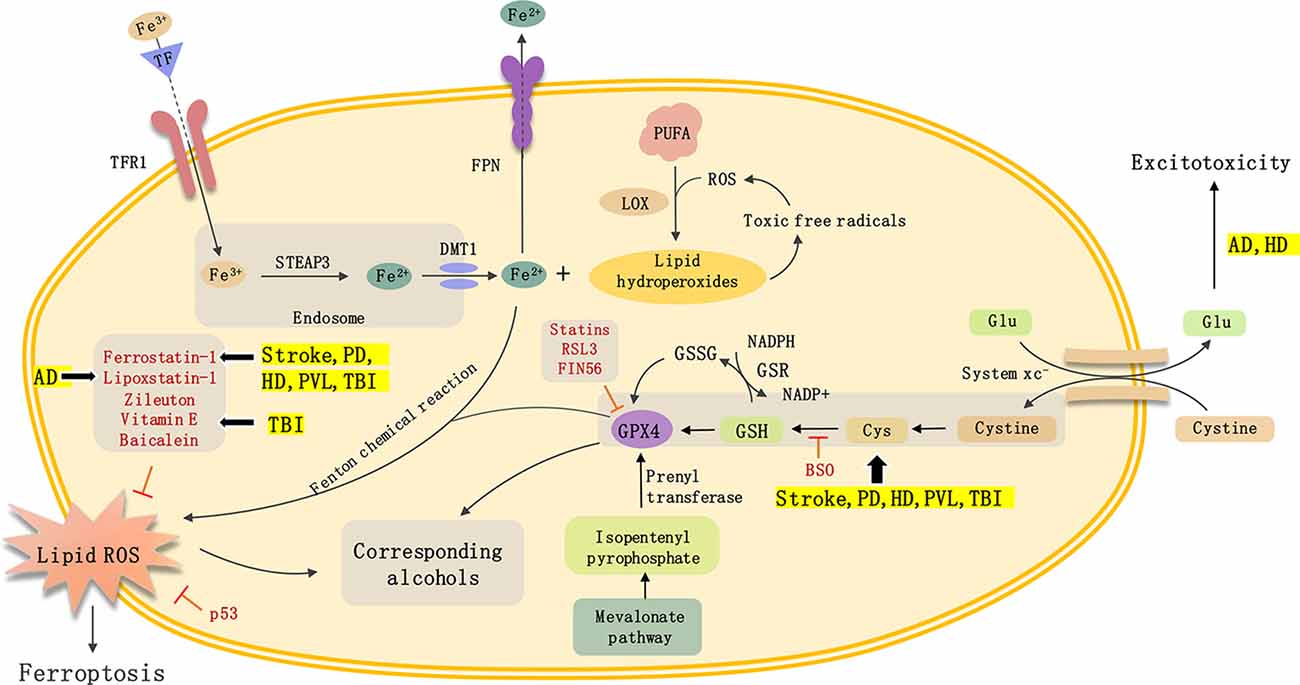
Free Fe3+ in the blood forms a complex with extracellular transferrin (Tf), which binds to transferrin receptor 1 (TfR1) on the cell membrane, and forms endosomes that are transported into the cell under endocytosis. In the cell, Fe3+ is catalyzed into Fe2+ by the enzyme’s six-transmembrane epithelial antigen of the prostate (STEAP3), which is transported from the endosome to the cytosol through the divalent metal ion transporter 1 (DMT1; Koskenkorva-Frank et al., 2013). Fe2+ can be pumped out through ferroportin, which is located on the cellular membrane or stored in ferritin in the cytoplasm to achieve intracellular iron homeostasis. When excess Fe2+ is produced in the cells, lipid ROS are generated by the Fenton chemical reaction, leading to the continuous accumulation of lipid ROS within the cell and the eventual development of ferroptosis (Cao and Dixon, 2016; Xie et al., 2016).
Lipid ROS Metabolism and Ferroptosis
The accumulation of lipid ROS is an important cause of ferroptosis, though the exact way it is produced remains to be elucidated (Muhoberac and Vidal, 2019; Stockwell and Jiang, 2020). ROS can interact with PUFAs on the lipid membrane to form lipid ROS. When a large amount of lipid ROS accumulates in the cell, it causes ferroptosis. Lipoxygenase is likely involved in the formation of iron-dependent lipid ROS, as it catalyzes the oxidation of PUFAs to lipid hydroperoxides. When numerous iron ions are present in the cytoplasm, lipid hydroperoxides form toxic lipid free radicals, causing cellular damage. In parallel, these free radicals can transfer protons near PUFAs, which starts a new round of lipid oxidation reaction and causes more serious oxidative damage (Nakamura et al., 2019; Su et al., 2019; Wang et al., 2020).
GPX4 Regulation and Ferroptosis
GPX4 is a class of antioxidant enzymes. GSH can degrade H2O2 and lipid ROS into H2O, and their corresponding alcohols, respectively, thus reducing intracellular lipid hydroperoxide, and organic hydroperoxide. These reactions cause cells to avoid oxidative damage, which is essential for cell survival (Imai et al., 2017; Ingold et al., 2018; Li et al., 2020).
GSH Synthesis Regulation Pathway and Ferroptosis
As GSH is a necessary co-factor for the function of GPX4, its synthesis directly affects GPX4 activity. The cystine/glutamate reverse transporter on the cell membrane, system xc−, transports glutamate to the outside of the cell and cystine into the cell. Once in the cytoplasm, cystine is transformed into cysteine (Cys), which is involved in the synthesis of reduced GSH (Massie et al., 2015). When system xc− transport cystine is blocked, the intracellular Cys levels are reduced, resulting in a decrease in GSH synthesis, a loss of GPX4 activity, intracellular lipid ROS accumulation, and ferroptosis. Inducers such as erastin can reduce intracellular Cys levels, inhibit GSH synthesis, and attenuate the accumulation of intracellular lipid ROS, leading to the development of ferroptosis by inhibiting the activity of system xc− (Dai et al., 2020; Wang et al., 2020).
Mevalonate Regulation Pathway
GPX4 is a selenium-containing protease, the activity of which is affected by the catalytic center selenocysteine (Sec). The genetic code of Sec is UGA, which is the same as the stop codon. Therefore, special transport RNA (tRNA[Ser]Sec) is required for this process (Wirth et al., 2014; Schweizer and Fradejas-Villar, 2016). The maturation process of tRNA[Ser]Sec, a single tRNA that transports Sec, requires prenylation modification at a specific adenine site. This is required for Sec to participate in the synthesis of selenoproteins. The prenyltransferase uses isopentenyl pyrophosphate (IPP) as the donor, which is an important product of the mevalonate (MVA) pathway. Therefore, the activity of GPX4 is regulated by MVA (Seibt et al., 2019). Interestingly, statins can hinder the maturation of tRNA[Ser]Sec, which affects the synthesis of GPX4, and causes ferroptosis (Yu et al., 2017).
Other Pathways
Other proteins regulate ferroptosis, such as the apoptosis molecule p53, which inhibits the activity of the transporter, reduces the intake of cystine and synthesis of GSH, and hinders the activity of GPX4 by inhibiting the expression of the system xc− subunit SLC7A11. p53 also reduces the capacity of the antioxidant effect and increases lipid ROS levels, leading to ferroptosis (Kang et al., 2019; Leu et al., 2019; Ye et al., 2019; Li et al., 2020a). Also, activation of the mitogen-activated protein kinase (MAPK) pathway can induce ferroptosis in cancer cells (Gao et al., 2018; Li et al., 2018; Su et al., 2019). Thus, in cancer cells with RAS mutations, blocking RAS/RAF/MEK signal can inhibit ferroptosis caused by erastin (Imai et al., 2017). However, the specific mechanism is still not clear.
Ferroptosis Inducer
Systeme xc−
Systeme xc− is a heterodimer composed of the transmembrane transporter SLC7A11 and regulatory protein SLC3A2L connected by disulfide bonds. This complex mediates cystine entry into cells and glutamate exit from the cells (Bridges et al., 2012; Massie et al., 2015). When the extracellular glutamate is present in excess, it inhibits the transfer of extra cysteine by systeme xc−, reduces intracellular Cys levels, and blocks the synthesis of GSH, resulting in the accumulation of intracellular lipid ROS and development of cell death (Tobaben et al., 2011; Kang et al., 2014; Yang and Stockwell, 2016). Previous studies have shown that after adding the ferroptosis inducer erastin, the level of intracellular radiolabeled Cys becomes significantly reduced, and the synthesis of GSH is blocked, subsequently causing ferroptosis (Dixon et al., 2012; Du et al., 2020). Sorafenib, a targeted therapeutic drug approved by the United States Food and Drug Administration (US FDA) for metastatic kidney cancer, can inhibit the activity of CRAF, BRAF, VEGFR, and PDGFR-β kinase in tumor cells (Hiles and Kolesar, 2008; Rini et al., 2020). Studies have shown that sorafenib can induce the occurrence of ferroptosis in tumor cells, opening up a novel method for tumor treatment (Sun et al., 2016; Xu et al., 2020). Sulfasalazine (SAS) is a sulfonamide antifungal drug that is widely used in the treatment of colitis. It can inhibit the expression of systeme xc− subunit SLC7A11 and reduce the levels of intracellular Cys, leading to the induction of ferroptosis in the pancreas, breast, head, or neck cancer (Kim et al., 2018; Yamaguchi et al., 2018; Yu et al., 2019; Shin et al., 2020). Glutamate can reduce intracellular Cys expression by inhibiting the transfer of systeme xc−, which leads to the inhibition of GSH synthesis and causes ferroptosis (Song et al., 2018; Wang et al., 2019).
GPX4 Inhibitor
While erastin acts on systeme xc−, it indirectly affects the synthesis of intracellular GSH, which, in turn, affects the activity of GPX4, causing the accumulation of intracellular lipid ROS and triggering ferroptosis (Yang et al., 2014; Hao et al., 2017). RAS selective lethal small molecule 3 (RSL3) cannot affect the synthesis of GSH in the cell, but can directly inhibit the target protein GPX4, resulting in loss of GPX4 activity and triggering ferroptosis (Sui et al., 2018; Ye et al., 2019; Vučković et al., 2020). The small molecule FIN56 promotes the degradation of the GPX4 protein and reduces the synthesis of the lipophilic antioxidant coenzyme Q 10 (CoQ 10) through the MVA pathway. This weakens the inhibitory effect of CoQ 10 on the production of lipid ROS, leading to the occurrence of ferroptosis. When GPX4 was overexpressed in cells, FIN56-induced cell death was inhibited (Shimada et al., 2016; Gaschler et al., 2018).
GSH Depleting Agent
As a reducing agent of GPX4, GSH directly affects the activity of GPX4 and induces ferroptosis when its synthesis is blocked. Previous studies have found that, in RAS mutant cells, buthionine sulfoximine (BSO) can inhibit the GPX4 synthesis, which reduces the synthesis of GSH, inhibits GPX4 activity, leads to the accumulation of intracellular lipid ROS, and causes ferroptosis (Nishizawa et al., 2018; Zhang et al., 2018).
Ferroptosis Inhibitor
Iron Chelating Agent
Iron chelating agents can turn free iron ions into stable compounds, thereby reducing its toxic effects and inhibiting the process of ferroptosis. Deferoxamine (DFO), a bacterial metabolite, is one type of iron-chelating agent that can bind to the six coordinated bonds in the center of the iron atom, reduce the Fe2+ level in the cytoplasm, and inhibit lipid ROS formation caused by Fe2+ accumulation. Ultimately, DFO inhibits the occurrence of ferroptosis (Louandre et al., 2013; Ma et al., 2016; Chen et al., 2017). Another chelating agent, ciclopirox (CPX), is a classical antifungal drug. The addition of CPX to neurons in vitro can inhibit glutamate-induced cell death (Ma et al., 2013).
Lipid ROS Inhibitor
Ferrostatin-1 is an aralkylamine-containing antioxidant that can lower levels of lipid ROS in the cytoplasm and inhibit ferroptosis induced by either erastin or RSL3 (Wenzel et al., 2017; Li et al., 2019). Similarly, lipoxstatin-1, which contains amide and sulfonamide subunits, can inhibit the lipid peroxidation pathway and the occurrence of ferroptosis by lowering lipid ROS levels in the cytoplasm (Skouta et al., 2014). Zileuton is a novel and selective 5-lipoxygenase inhibitor that can prevent the production of ROS in the cytoplasm and effectively prevent glutamate-induced ferroptosis in mouse hippocampal neuronal cells (Liu et al., 2015; Yuan et al., 2016). As a well-known lipid ROS inhibitor, vitamin E can reduce cell death by inhibiting the lipid oxidation pathway, thereby attenuating neurological dysfunction (Carlson et al., 2016; Imai et al., 2017; Hinman et al., 2018).
Ferroptosis and Nervous System Diseases
Statistics show that the number of patients with neurological diseases such as stroke and neurodegenerative disease increases year by year, thus placing a great burden on families and society. However, due to the unclear pathogenesis of these diseases and a lack of reliable diagnostic and treatment methods, a greater in-depth research is urgently needed. Previous studies on ferroptosis have focused on the field of cancer. In recent years, researchers have found that ferroptosis can also exert a significant effect on neurological diseases (Yan and Zhang, 2019; Yan et al., 2020).
Traumatic Brain Injury and Ferroptosis
Traumatic brain injury (TBI) is a leading cause of disability and mortality, with 1.5 million hospital admissions and 57,000 deaths in Europe each year. Acute brain injuries resulting from TBI can lead to lasting neurologic and cognitive problems. Mechanisms accounting for brain damage after TBI include biophysical forces, neuropathological changes (e.g., multifocal axonal injury, microglial activation, and microhemorrhages) and pathological responses (e.g., inflammation, mitochondrial dysfunction, and oxidative stress). Therapeutic strategies for TBI include pharmacological agents (e.g., anti-inflammatory agents and cell cycle inhibitors), noninvasive approaches (e.g., exercise therapy and transcranial magnetic stimulation), and biologics. Recent studies have shown that ferroptosis may play a role in TBI. After establishing a controlled cortical impact injury (CCI) mouse model, Xie et al. (2016) observed iron accumulation, reduced GPx activity, and increased lipid ROS after TBI. They also found that the administration of Ferrostatin-1 by cerebral ventricular injection reduced ferroptosis and attenuated injury lesions. The role of ferroptosis in TBI and the neuroprotective function of baicalein were supported by Li et al. (2020a) who conducted in vivo and in vitro studies and also showed that baicalein had neuroprotective effects against posttraumatic epileptic seizures. Xie et al. (2016) detected the expression of ferroptosis-related molecules at 6 h, 12 h, 24 h, 48 h, and 72 h following CCI in mice. They reported that overexpression of miR-212-5p reduced ferroptosis while downregulation of miR-212-5p induced ferroptosis partially by targeting prostaglandin-endoperoxide synthase-2. Moreover, the administration of miR-212-5p significantly improved learning and spatial memory in CCI mice. Therefore, ferroptosis participates in the pathological process of TBI, and inhibiting ferroptotic death via different pathways may have neuroprotective effects. Additionally, due to a damaged blood-brain barrier and impaired homeostasis, TBI can contribute to secondary injury that occurs from hours to months. The delayed period suggests a potential therapeutic window intervention. However, because of limited data, the time window is under exploration and more studies on ferroptosis associated treatment for TBI are needed.
Stroke and Ferroptosis
Stroke has high morbidity, high lethality, and a high disability rate. Stroke can be classified into either hemorrhagic or ischemic stroke. Ischemic stroke develops due to carotid and vertebral artery stenosis or occlusion, causing the insufficient blood supply to the brain. This results in a depletion of oxygen and nutrients within the brain, leading to oxidative stress, mitochondrial damage, and ultimately, cell death (Andrabi et al., 2020; Datta et al., 2020). Previous studies have found that ferroptosis is closely related to ischemic stroke (Ahmad et al., 2014) found that in a mouse ischemic stroke model, GSH was significantly reduced, and the levels of lipid ROS were increased. Other scholars report that ferroptosis can cause neuronal death after ischemic stroke and that an inhibitor of ferrostatin-1 or liproxatin-1 plays a protective role in the mouse ischemic stroke model (Guan et al., 2019; Lan et al., 2020). Li et al. (2020a) analyzed the ultrastructure of tissue samples from patients with cerebral hemorrhage using transmission electron microscopy and observed that ferroptosis coexists with apoptosis, necrosis, and autophagy (Nikoletopoulou et al., 2013; Li et al., 2018). The combined use of inhibitors that target multiple avenues of cell death is better than the use of a certain form of death inhibitor in reducing neuronal damage (Weiland et al., 2019). Previous studies have found that selenium ions can enhance the expression of GPX4 and inhibit ferroptosis by activating the transcription factors TFAP2c and Sp1 (Ingold et al., 2018; Alim et al., 2019; Conrad and Proneth, 2020). Treatment of selenium ions can enhance the expression of the antioxidant GPX4, protect from neuronal injury, and improve behavioral defects in mouse models of stroke (Alim et al., 2019). Li et al. (2020b) found that inhibition of ferroptosis alleviates early brain injury after subarachnoid hemorrhage in vitro and in vivo, which is associated with a reduction of lipid peroxidation. Therefore, suppressing ferroptosis can be used as an effective treatment for stroke.
Neurodegenerative Disease and Ferroptosis
Alzheimer’s Disease and Ferroptosis
Alzheimer’s disease (AD) is a familial neurodegenerative disease. The clinical manifestations include cognitive impairment, memory decline, executive ability decline, and personality changes, among others. The pathological features of AD include the presence of senile plaques that are formed by extracellular β-amyloid deposition, nerve fiber tangles formed by abnormal phosphorylation of Tau protein, neuronal damage, and abnormal synaptic function (Ballard et al., 2011; Palop and Mucke, 2016). Characteristics associated with ferroptosis can be detected in the brains of AD patients and mice, such as iron metabolism disorder, glutamate excitotoxicity, and lipid ROS accumulation. Numerous studies show that ferritin in the brain of AD patients is related to the content of apolipoprotein in the cerebrospinal fluid. Increased ferritin is accompanied by an up-regulation in the expression of the AD risk gene APOE-ε4, indicating that the iron levels in the brain affect the AD process and that high levels of iron in the brain can be used as a risk factor of AD (Ayton et al., 2015; Xu et al., 2016). Additionally, glutamate excitotoxicity is involved in the pathogenesis of AD. Dysfunction of systeme xc− during ferroptosis can lead to an increase in the concentration of extracellular glutamate, causing excitotoxicity (Kang et al., 2018; Zille et al., 2019; Zhang et al., 2020). Furthermore, oxidative stress is critically related to AD. Recent studies have shown that oxidative stress can promote oligomeric Aβ disorder and tau protein tangles-induced neurotoxicity (Jiang et al., 2016; Luengo et al., 2019). Accumulation of lipid ROS during ferroptosis can cause oxidative damage to cells, resulting in neuronal damage and the development of AD (Xie et al., 2016; Wu et al., 2018). Hambright et al. (2017) found that a knockout of GPX4 in the mouse brain neurons caused neuronal degeneration, accompanied by cognitive dysfunction. However, administration of the ferroptosis inhibitor, Liproxstatin-1, can reverse the neuronal degeneration and improve the cognitive function of mice (Hambright et al., 2017).
Parkinson’s Disease and Ferroptosis
Parkinson’s disease (PD) is a degenerative neurological disease that is mainly observed in the elderly population. The clinical manifestations include resting tremor, bradykinesia, muscle stiffness, and posture and gait disorders, accompanied by memory loss, mental decline, and emotional disorders. The pathological features of the disease mainly include degeneration and death of midbrain substantia nigra dopaminergic (DA) neurons, reduced striatum DA concentration, and formation of Lewy bodies (Goedert and Compston, 2018; Homayoun, 2018). Recently, researchers found that in PD patients, iron and hydroxyl radical levels in the substantia nigra are increased, leading to damage of DA neurons. Besides, other ferroptosis characteristics such as GSH depletion have also been observed in the substantia nigra of PD patients (Zhao, 2019; Devos et al., 2020; Zhang et al., 2020). Another study reported that loss of plasma ceruloplasmin iron oxidase activity in the substantia nigra of PD patients, which led to the accumulation of iron peroxide. Interestingly, iron chelating agents were able to reverse the accumulation of iron ions caused by the absence of ceruloplasmin, improve the exercise ability of PD mice, and effectively reduce any neurological damage caused by MPTP (Ayton et al., 2013), consistent with prior results performed in PD patients (Grolez et al., 2015). Do Van et al. (2016) and Dächert et al. (2020) confirmed using in vitro brain slice examination and in vivo MPTP mouse model that PKCα activation caused MEK activation, which, in turn, caused ferroptosis. Therefore, iron chelators, ferrostatin-1, and PKC inhibitors, which regulate ferroptosis, may represent novel drugs for PD patients.
Huntington’s Disease and Ferroptosis
Huntington’s disease (HD) is a type of inherited neurological disease caused by repeated amplification of CAG in the HTT gene. The clinical manifestations of HD are involuntary dance-like movements, dementia, and mood disorders (Roos, 2010; Wyant et al., 2017). Ferroptosis is related to the excitotoxicity of glutamate and GSH-mediated redox reactions in HD. Abnormal levels of glutamate, GSH, iron ions, and accumulation of intracellular lipid ROS have also been detected in HD patients (Dubinsky, 2017; Kumar et al., 2020). In animal HD models, characteristics of ferroptosis, including blocked GSH synthesis and decreased activity of GPXs, have been detected (Ribeiro et al., 2012; Mason et al., 2013). Treatment of HD rats using the ferroptosis inhibitor ferrostatin-1 was found to inhibit lipid peroxidation and reduce neuronal death. However, the use of iron chelating agents can reverse the changes of lipid peroxidation inhibition and neuronal death reduction, and significantly improve cognitive dysfunction of HD rats (Chen et al., 2013; Skouta et al., 2014; Agrawal et al., 2018).
Periventricular Leukomalacia and Ferroptosis
Periventricular leukomalacia (PVL), a secondary brain white matter injury, is commonly seen in premature infants and surviving children with postpartum asphyxia. Hypoxia before and after the perinatal period causes ischemia of the peripheral ventricle region of the child, resulting in damage to neurons and oligodendrocytes (OLs) and abnormal formation of the myelin sheath. Hypoxia also causes softening of the white matter around the ventricles, resulting in bilateral spastic hemiplegia, quadriplegia, and mental retardation (Deng et al., 2008; Back, 2017). Indirect evidence has indicated that ferroptosis may play a key role in the development of PVL. Inder et al. (2002) found a large number of lipid oxidation products in the cerebrospinal fluid of infants with PVL. Furthermore, OLs death in PVL is related to lipid peroxidation, which is one of the key characteristics of ferroptosis. These results suggest that ferroptosis may be involved in the pathogenesis of PVL (Inder et al., 2002). Similarly, other studies that used cultivated OLs in cysteine-free medium found that exhaustion of GSH caused ferroptosis in cells, and the administration of ferrostatin-1 can effectively prevent ferroptosis in OLs (Novgorodov et al., 2018; Nobuta et al., 2019).
Conclusions and Prospects
In this review article, we mainly describe the role of ferroptosis in non-neoplastic neurological diseases, including TBI, stroke, neurodegenerative disease, and PVL (Figure 1). And summarize the inducer and inhibitor of ferroptosis used in previous research. Ferroptosis is an iron-dependent form of cell death characterized by the accumulation of intracellular lipid ROS. Ferroptosis is closely related to the progress of various neurological diseases including AD, PD, HD, and stroke. However, there are still many issues in this field that need to be resolved. First, researchers need to examine the relationship between ferroptosis and other cell death types such as apoptosis, autophagy, and necrosis. Second, the similarities and differences in the molecular mechanism of ferroptosis across different disease states need to be better defined. Third, there needs to be further research on drugs that target ferroptosis to determine whether they can play an important role in the clinical treatment of neurological diseases. In-depth research on ferroptosis will help us further clarify the pathogenesis of neurological diseases and provide a theoretical basis for clinical treatment targeting ferroptosis to prevent and treat neurological diseases.
Author Contributions
All authors participated in analyzing and discussing the literature, commenting on, and approving the manuscript. JZ supervised the research, led the discussion, wrote and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Agrawal, S., Fox, J., Thyagarajan, B., and Fox, J. H. (2018). Brain mitochondrial iron accumulates in Huntington’s disease, mediates mitochondrial dysfunction, and can be removed pharmacologically. Free Radic. Biol. Med. 120, 317–329. doi: 10.1016/j.freeradbiomed.2018.04.002
Ahmad, S., Elsherbiny, N. M., Haque, R., Khan, M. B., Ishrat, T., Shah, Z. A., et al. (2014). Sesamin attenuates neurotoxicity in mouse model of ischemic brain stroke. Neurotoxicology 45, 100–110. doi: 10.1016/j.neuro.2014.10.002
Alim, I., Caulfield, J. T., Chen, Y., Swarup, V., Geschwind, D. H., Ivanova, E., et al. (2019). Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell 177, 1262.e25–1279.e25. doi: 10.1016/j.cell.2019.03.032
Andrabi, S. S., Parvez, S., and Tabassum, H. (2020). Ischemic stroke and mitochondria: mechanisms and targets. Protoplasma 257, 335–343. doi: 10.1007/s00709-019-01439-2
Ayton, S., Faux, N. G., Bush, A. I., and Alzheimer’s Disease Neuroimaging Initiative. (2015). Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by APOE. Nat. Commun. 6:6760. doi: 10.1038/ncomms7760
Ayton, S., Lei, P., Duce, J. A., Wong, B. X., Sedjahtera, A., Adlard, P. A., et al. (2013). Ceruloplasmin dysfunction and therapeutic potential for Parkinson disease. Ann. Neurol. 73, 554–559. doi: 10.1002/ana.23817
Back, S. A. (2017). White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol. 134, 331–349. doi: 10.1007/s00401-017-1718-6
Ballard, C., Gauthier, S., Corbett, A., Brayne, C., Aarsland, D., and Jones, E. (2011). Alzheimer’s disease. Lancet 377, 1019–1031. doi: 10.1016/S0140-6736(10)61349-9
Bridges, R., Lutgen, V., Lobner, D., and Baker, D. A. (2012). Thinking outside the cleft to understand synaptic activity: contribution of the cystine-glutamate antiporter (System xc-) to normal and pathological glutamatergic signaling. Pharmacol. Rev. 64, 780–802. doi: 10.1124/pr.110.003889
Cao, J. Y., and Dixon, S. J. (2016). Mechanisms of ferroptosis. Cell. Mol. Life Sci. 73, 2195–2209. doi: 10.1007/s00018-016-2194-1
Carlson, B. A., Tobe, R., Yefremova, E., Tsuji, P. A., Hoffmann, V. J., Schweizer, U., et al. (2016). Glutathione peroxidase 4 and vitamin E cooperatively prevent hepatocellular degeneration. Redox Biol. 9, 22–31. doi: 10.1016/j.redox.2016.05.003
Chen, J., Marks, E., Lai, B., Zhang, Z., Duce, J. A., Lam, L. Q., et al. (2013). Iron accumulates in Huntington’s disease neurons: protection by deferoxamine. PLoS One 8:e77023. doi: 10.1371/journal.pone.0077023
Chen, M. S., Wang, S. F., Hsu, C. Y., Yin, P. H., Yeh, T. S., Lee, H. C., et al. (2017). CHAC1 degradation of glutathione enhances cystine-starvation-induced necroptosis and ferroptosis in human triple negative breast cancer cells via the GCN2-eIF2α-ATF4 pathway. Oncotarget 8, 114588–114602. doi: 10.18632/oncotarget.23055
Conrad, M., and Proneth, B. (2020). Selenium: tracing another essential element of ferroptotic cell death. Cell Chem. Biol. 27, 409–419. doi: 10.1016/j.chembiol.2020.03.012
Dächert, J., Ehrenfeld, V., Habermann, K., Dolgikh, N., and Fulda, S. (2020). Targeting ferroptosis in rhabdomyosarcoma cells. Int. J. Cancer 146, 510–520. doi: 10.1002/ijc.32496
Dai, C., Chen, X., Li, J., Comish, P., Kang, R., and Tang, D. (2020). Transcription factors in ferroptotic cell death. Cancer Gene Ther. doi: 10.1038/s41417-020-0170-2 [Epub ahead of print].
Datta, A., Sarmah, D., Mounica, L., Kaur, H., Kesharwani, R., Verma, G., et al. (2020). Cell death pathways in ischemic stroke and targeted pharmacotherapy. Transl. Stroke Res. doi: 10.1007/s12975-020-00806-z [Epub ahead of print].
Deng, W., Pleasure, J., and Pleasure, D. (2008). Progress in periventricular leukomalacia. Arch. Neurol. 65, 1291–1295. doi: 10.1001/archneur.65.10.1291
Derry, P. J., Hegde, M. L., Jackson, G. R., Kayed, R., Tour, J. M., Tsai, A. L., et al. (2020). Revisiting the intersection of amyloid, pathologically modified tau and iron in Alzheimer’s disease from a ferroptosis perspective. Prog. Neurobiol. 184:101716. doi: 10.1016/j.pneurobio.2019.101716
Devos, D., Cabantchik, Z. I., Moreau, C., Danel, V., Mahoney-Sanchez, L., Bouchaoui, H., et al. (2020). Conservative iron chelation for neurodegenerative diseases such as Parkinson’s disease and amyotrophic lateral sclerosis. J. Neural Transm. 127, 189–203. doi: 10.1007/s00702-019-02138-1
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., Skouta, R., Zaitsev, E. M., Gleason, C. E., et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072. doi: 10.1016/j.cell.2012.03.042
Do Van, B., Gouel, F., Jonneaux, A., Timmerman, K., Gele, P., Petrault, M., et al. (2016). Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC. Neurobiol. Dis. 94, 169–178. doi: 10.1016/j.nbd.2016.05.011
Dolma, S., Lessnick, S. L., Hahn, W. C., and Stockwell, B. R. (2003). Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3, 285–296. doi: 10.1016/s1535-6108(03)00050-3
Du, J., Zhou, Y., Li, Y., Xia, J., Chen, Y., Chen, S., et al. (2020). Identification of Frataxin as a regulator of ferroptosis. Redox Biol. 32:101483. doi: 10.1016/j.redox.2020.101483
Dubinsky, J. M. (2017). Towards an understanding of energy impairment in Huntington’s disease brain. J. Huntingtons Dis. 6, 267–302. doi: 10.3233/jhd-170264
Gao, H., Bai, Y., Jia, Y., Zhao, Y., Kang, R., Tang, D., et al. (2018). Ferroptosis is a lysosomal cell death process. Biochem. Biophys. Res. Commun. 503, 1550–1556. doi: 10.1016/j.bbrc.2018.07.078
Gaschler, M. M., Andia, A. A., Liu, H., Csuka, J. M., Hurlocker, B., Vaiana, C. A., et al. (2018). FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat. Chem. Biol. 14, 507–515. doi: 10.1038/s41589-018-0031-6
Goedert, M., and Compston, A. (2018). Parkinson’s disease—the story of an eponym. Nat. Rev. Neurol. 14, 57–62. doi: 10.1038/nrneurol.2017.165
Grolez, G., Moreau, C., Sablonniere, B., Garcon, G., Devedjian, J. C., Meguig, S., et al. (2015). Ceruloplasmin activity and iron chelation treatment of patients with Parkinson’s disease. BMC Neurol. 15:74. doi: 10.1186/s12883-015-0331-3
Guan, X., Li, X., Yang, X., Yan, J., Shi, P., Ba, L., et al. (2019). The neuroprotective effects of carvacrol on ischemia/reperfusion-induced hippocampal neuronal impairment by ferroptosis mitigation. Life Sci. 235:116795. doi: 10.1016/j.lfs.2019.116795
Hambright, W. S., Fonseca, R. S., Chen, L., Na, R., and Ran, Q. (2017). Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 12, 8–17. doi: 10.1016/j.redox.2017.01.021
Hao, S., Yu, J., He, W., Huang, Q., Zhao, Y., Liang, B., et al. (2017). Cysteine dioxygenase 1 mediates erastin-induced ferroptosis in human gastric cancer cells. Neoplasia 19, 1022–1032. doi: 10.1016/j.neo.2017.10.005
Hiles, J. J., and Kolesar, J. M. (2008). Role of sunitinib and sorafenib in the treatment of metastatic renal cell carcinoma. Am J. Health Syst. Pharm. 65, 123–131. doi: 10.2146/ajhp060661
Hinman, A., Holst, C. R., Latham, J. C., Bruegger, J. J., Ulas, G., McCusker, K. P., et al. (2018). Vitamin E hydroquinone is an endogenous regulator of ferroptosis via redox control of 15-lipoxygenase. PLoS One 13:e0201369. doi: 10.1371/journal.pone.0201369
Homayoun, H. (2018). Parkinson disease. Ann. Intern. Med. 169, ITC33–ITC48. doi: 10.7326/AITC201809040
Imai, H., Matsuoka, M., Kumagai, T., Sakamoto, T., and Koumura, T. (2017). Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr. Top. Microbiol. Immunol. 403, 143–170. doi: 10.1007/82_2016_508
Inder, T., Mocatta, T., Darlow, B., Spencer, C., Volpe, J. J., and Winterbourn, C. (2002). Elevated free radical products in the cerebrospinal fluid of VLBW infants with cerebral white matter injury. Pediatr. Res. 52, 213–218. doi: 10.1203/00006450-200208000-00013
Ingold, I., Berndt, C., Schmitt, S., Doll, S., Poschmann, G., Buday, K., et al. (2018). Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 172, 409.e21–422.e21. doi: 10.1016/j.cell.2017.11.048
Jiang, T., Sun, Q., and Chen, S. (2016). Oxidative stress: a major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog. Neurobiol. 147, 1–19. doi: 10.1016/j.pneurobio.2016.07.005
Kang, R., Kroemer, G., and Tang, D. (2019). The tumor suppressor protein p53 and the ferroptosis network. Free Radic. Biol. Med. 133, 162–168. doi: 10.1016/j.freeradbiomed.2018.05.074
Kang, Y., Tiziani, S., Park, G., Kaul, M., and Paternostro, G. (2014). Cellular protection using Flt3 and PI3Kα inhibitors demonstrates multiple mechanisms of oxidative glutamate toxicity. Nat. Commun. 5:3672. doi: 10.1038/ncomms4672
Kang, R., Zhu, S., Zeh, H. J., Klionsky, D. J., and Tang, D. (2018). BECN1 is a new driver of ferroptosis. Autophagy 14, 2173–2175. doi: 10.1080/15548627.2018.1513758
Kim, E. H., Shin, D., Lee, J., Jung, A. R., and Roh, J. L. (2018). CISD2 inhibition overcomes resistance to sulfasalazine-induced ferroptotic cell death in head and neck cancer. Cancer Lett. 432, 180–190. doi: 10.1016/j.canlet.2018.06.018
Koskenkorva-Frank, T. S., Weiss, G., Koppenol, W. H., and Burckhardt, S. (2013). The complex interplay of iron metabolism, reactive oxygen species and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic. Biol. Med. 65, 1174–1194. doi: 10.1016/j.freeradbiomed.2013.09.001
Kumar, A., Kumar, V., Singh, K., Kumar, S., Kim, Y. S., Lee, Y. M., et al. (2020). Therapeutic advances for Huntington’s disease. Brain Sci. 10:43. doi: 10.3390/brainsci10010043
Lan, B., Ge, J. W., Cheng, S. W., Zheng, X. L., Liao, J., He, C., et al. (2020). Extract of naotaifang, a compound chinese herbal medicine, protects neuron ferroptosis induced by acute cerebral ischemia in rats. J. Integr. Med. doi: 10.1016/j.joim.2020.01.008 [Epub ahead of print].
Leu, J. I., Murphy, M. E., and George, D. L. (2019). Mechanistic basis for impaired ferroptosis in cells expressing the African-centric S47 variant of p53. Proc. Natl. Acad. Sci. U S A 116, 8390–8396. doi: 10.1073/pnas.1821277116
Li, Y., Cao, Y., Xiao, J., Shang, J., Tan, Q., Ping, F., et al. (2020a). Inhibitor of apoptosis-stimulating protein of p53 inhibits ferroptosis and alleviates intestinal ischemia/reperfusion-induced acute lung injury. Cell Death Differ. doi: 10.1038/s41418-020-0528-x [Epub ahead of print].
Li, Y., Liu, Y., Wu, P., Tian, Y., Liu, B., Wang, J., et al. (2020b). Inhibition of ferroptosis alleviates early brain injury after subarachnoid hemorrhage in vitro and in vivo via reduction of lipid peroxidation. Cell. Mol. Neurobiol. doi: 10.1007/s10571-020-00850-1 [Epub ahead of print].
Li, W., Feng, G., Gauthier, J. M., Lokshina, I., Higashikubo, R., Evans, S., et al. (2019). Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation. J. Clin. Invest. 129, 2293–2304. doi: 10.1172/jci126428
Li, L., Hao, Y., Zhao, Y., Wang, H., Zhao, X., Jiang, Y., et al. (2018). Ferroptosis is associated with oxygen-glucose deprivation/reoxygenation-induced Sertoli cell death. Int. J. Mol. Med. 41, 3051–3062. doi: 10.3892/ijmm.2018.3469
Li, Q., Weiland, A., Chen, X., Lan, X., Han, X., Durham, F., et al. (2018). Ultrastructural characteristics of neuronal death and white matter injury in mouse brain tissues after intracerebral hemorrhage: coexistence of ferroptosis, autophagy, and necrosis. Front. Neurol. 9:581. doi: 10.3389/fneur.2018.00581
Li, C., Zhang, Y., Liu, J., Kang, R., Klionsky, D. J., and Tang, D. (2020). Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy doi: 10.1080/15548627.2020.1739447 [Epub ahead of print].
Liang, C., Zhang, X., Yang, M., and Dong, X. (2019). Recent progress in ferroptosis inducers for cancer therapy. Adv. Mater. 31:e1904197. doi: 10.1002/adma.201904197
Liu, Y., Wang, W., Li, Y., Xiao, Y., Cheng, J., and Jia, J. (2015). The 5-lipoxygenase inhibitor zileuton confers neuroprotection against glutamate oxidative damage by inhibiting ferroptosis. Biol. Pharm. Bull. 38, 1234–1239. doi: 10.1248/bpb.b15-00048
Louandre, C., Ezzoukhry, Z., Godin, C., Barbare, J. C., Maziere, J. C., Chauffert, B., et al. (2013). Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int. J. Cancer 133, 1732–1742. doi: 10.1002/ijc.28159
Luengo, E., Buendia, I., Fernandez-Mendivil, C., Trigo-Alonso, P., Negredo, P., Michalska, P., et al. (2019). Pharmacological doses of melatonin impede cognitive decline in tau-related Alzheimer models, once tauopathy is initiated, by restoring the autophagic flux. J. Pineal Res. 67:e12578. doi: 10.1111/jpi.12578
Ma, S., Henson, E. S., Chen, Y., and Gibson, S. B. (2016). Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 7:e2307. doi: 10.1038/cddis.2016.208
Ma, T. C., Langley, B., Ko, B., Wei, N., Gazaryan, I. G., Zareen, N., et al. (2013). A screen for inducers of p21(waf1/cip1) identifies HIF prolyl hydroxylase inhibitors as neuroprotective agents with antitumor properties. Neurobiol. Dis. 49, 13–21. doi: 10.1016/j.nbd.2012.08.016
Mason, R. P., Casu, M., Butler, N., Breda, C., Campesan, S., Clapp, J., et al. (2013). Glutathione peroxidase activity is neuroprotective in models of Huntington’s disease. Nat. Genet. 45, 1249–1254. doi: 10.1038/ng.2732
Massie, A., Boillée, S., Hewett, S., Knackstedt, L., and Lewerenz, J. (2015). Main path and byways: non-vesicular glutamate release by system xc(-) as an important modifier of glutamatergic neurotransmission. J. Neurochem. 135, 1062–1079. doi: 10.1111/jnc.13348
Mizushima, N., and Komatsu, M. (2011). Autophagy: renovation of cells and tissues. Cell 147, 728–741. doi: 10.1016/j.cell.2011.10.026
Muhoberac, B. B., and Vidal, R. (2019). Iron, ferritin, hereditary ferritinopathy, and neurodegeneration. Front. Neurosci. 13:1195. doi: 10.3389/fnins.2019.01195
Nakamura, T., Naguro, I., and Ichijo, H. (2019). Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim. Biophys. Acta Gen. Subj. 1863, 1398–1409. doi: 10.1016/j.bbagen.2019.06.010
Nikoletopoulou, V., Markaki, M., Palikaras, K., and Tavernarakis, N. (2013). Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta 1833, 3448–3459. doi: 10.1016/j.bbamcr.2013.06.001
Nishizawa, S., Araki, H., Ishikawa, Y., Kitazawa, S., Hata, A., Soga, T., et al. (2018). Low tumor glutathione level as a sensitivity marker for glutamate-cysteine ligase inhibitors. Oncol. Lett. 15, 8735–8743. doi: 10.3892/ol.2018.8447
Nobuta, H., Yang, N., Ng, Y. H., Marro, S. G., Sabeur, K., Chavali, M., et al. (2019). Oligodendrocyte death in pelizaeus-merzbacher disease is rescued by iron chelation. Cell Stem Cell 25, 531.e6–541.e6. doi: 10.1016/j.stem.2019.09.003
Novgorodov, S. A., Voltin, J. R., Gooz, M. A., Li, L., Lemasters, J. J., and Gudz, T. I. (2018). Acid sphingomyelinase promotes mitochondrial dysfunction due to glutamate-induced regulated necrosis. J. Lipid. Res. 59, 312–329. doi: 10.1194/jlr.m080374
Palop, J. J., and Mucke, L. (2016). Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 17, 777–792. doi: 10.1038/nrn.2016.141
Ribeiro, M., Rosenstock, T. R., Cunha-Oliveira, T., Ferreira, I. L., Oliveira, C. R., and Rego, A. C. (2012). Glutathione redox cycle dysregulation in Huntington’s disease knock-in striatal cells. Free Radic. Biol. Med. 53, 1857–1867. doi: 10.1016/j.freeradbiomed.2012.09.004
Rini, B. I., Pal, S. K., Escudier, B. J., Atkins, M. B., Hutson, T. E., Porta, C., et al. (2020). Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol. 21, 95–104. doi: 10.1016/s1470-2045(19)30735-1
Roos, R. A. C. (2010). Huntington’s disease: a clinical review. Orphanet. J. Rare Dis. 5:40. doi: 10.1186/1750-1172-5-40
Schweizer, U., and Fradejas-Villar, N. (2016). Why 21? The significance of selenoproteins for human health revealed by inborn errors of metabolism. FASEB J. 30, 3669–3681. doi: 10.1096/fj.201600424
Seibt, T. M., Proneth, B., and Conrad, M. (2019). Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic. Biol. Med. 133, 144–152. doi: 10.1016/j.freeradbiomed.2018.09.014
Shimada, K., Skouta, R., Kaplan, A., Yang, W. S., Hayano, M., Dixon, S. J., et al. (2016). Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat. Chem. Biol. 12, 497–503. doi: 10.1038/nchembio.2079
Shin, D., Lee, J., You, J. H., Kim, D., and Roh, J. L. (2020). Dihydrolipoamide dehydrogenase regulates cystine deprivation-induced ferroptosis in head and neck cancer. Redox Biol. 30:101418. doi: 10.1016/j.redox.2019.101418
Skouta, R., Dixon, S. J., Wang, J., Dunn, D. E., Orman, M., Shimada, K., et al. (2014). Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J. Am. Chem. Soc. 136, 4551–4556. doi: 10.1021/ja411006a
Song, X., Zhu, S., Chen, P., Hou, W., Wen, Q., Liu, J., et al. (2018). AMPK-mediated BECN1 phosphorylation promotes ferroptosis by directly blocking system Xc− activity. Curr. Biol. 28, 2388.e5–2399.e5. doi: 10.1016/j.cub.2018.05.094
Stockwell, B. R., Friedmann Angeli, J. P., Bayir, H., Bush, A. I., Conrad, M., Dixon, S. J., et al. (2017). Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285. doi: 10.1016/j.cell.2017.09.021
Stockwell, B. R., and Jiang, X. (2020). The chemistry and biology of ferroptosis. Cell Chem. Biol. 27, 365–375. doi: 10.1016/j.chembiol.2020.03.013
Su, L., Jiang, X., Yang, C., Zhang, J., Chen, B., Li, Y., et al. (2019). Pannexin 1 mediates ferroptosis that contributes to renal ischemia/reperfusion injury. J. Biol. Chem. 294, 19395–19404. doi: 10.1074/jbc.RA119.010949
Su, L. J., Zhang, J. H., Gomez, H., Murugan, R., Hong, X., Xu, D., et al. (2019). Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy and ferroptosis. Oxid. Med. Cell. Longev. 2019:5080843. doi: 10.1155/2019/5080843
Sui, X., Zhang, R., Liu, S., Duan, T., Zhai, L., Zhang, M., et al. (2018). RSL3 drives ferroptosis through GPX4 inactivation and ROS production in colorectal cancer. Front. Pharmacol. 9:1371. doi: 10.3389/fphar.2018.01371
Sun, X., Niu, X., Chen, R., He, W., Chen, D., Kang, R., et al. (2016). Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology 64, 488–500. doi: 10.1002/hep.28574
Tobaben, S., Grohm, J., Seiler, A., Conrad, M., Plesnila, N., and Culmsee, C. (2011). Bid-mediated mitochondrial damage is a key mechanism in glutamate-induced oxidative stress and AIF-dependent cell death in immortalized HT-22 hippocampal neurons. Cell Death Differ. 18, 282–392. doi: 10.1038/cdd.2010.92
Vučković, A. M., Bosello Travain, V., Bordin, L., Cozza, G., Miotto, G., Rossetto, M., et al. (2020). Inactivation of the glutathione peroxidase GPx4 by the ferroptosis-inducing molecule RSL3 requires the adaptor protein 14–3-3ε. FEBS Lett. 594, 611–624. doi: 10.1002/1873-3468.13631
Wallach, D., Kang, T. B., Dillon, C. P., and Green, D. R. (2016). Programmed necrosis in inflammation: toward identification of the effector molecules. Science 352:aaf2154. doi: 10.1126/science.aaf2154
Wang, H., Liu, C., Zhao, Y., and Gao, G. (2020). Mitochondria regulation in ferroptosis. Eur. J. Cell Biol. 99:151058. doi: 10.1016/j.ejcb.2019.151058
Wang, L., Liu, Y., Du, T., Yang, H., Lei, L., Guo, M., et al. (2020). ATF3 promotes erastin-induced ferroptosis by suppressing system Xc. Cell Death Differ. 27, 662–675. doi: 10.1038/s41418-019-0380-z
Wang, W., Green, M., Choi, J. E., Gijon, M., Kennedy, P. D., Johnson, J. K., et al. (2019). CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569, 270–274. doi: 10.1038/s41586-019-1170-y
Weiland, A., Wang, Y., Wu, W., Lan, X., Han, X., Li, Q., et al. (2019). Ferroptosis and its role in diverse brain diseases. Mol. Neurobiol. 56, 4880–4893. doi: 10.1007/s12035-018-1403-3
Wenzel, S. E., Tyurina, Y. Y., Zhao, J., St Croix, C. M., Dar, H. H., Mao, G., et al. (2017). PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell 171, 628.e26–641.e26. doi: 10.1016/j.cell.2017.09.044
Williams, G. T., and Smith, C. A. (1993). Molecular regulation of apoptosis: genetic controls on cell death. Cell 74, 777–779. doi: 10.1016/0092-8674(93)90457-2
Wirth, E. K., Bharathi, B. S., Hatfield, D., Conrad, M., Brielmeier, M., and Schweizer, U. (2014). Cerebellar hypoplasia in mice lacking selenoprotein biosynthesis in neurons. Biol. Trace. Elem. Res. 158, 203–210. doi: 10.1007/s12011-014-9920-z
Wu, J. R., Tuo, Q. Z., and Lei, P. (2018). Ferroptosis, a recent defined form of critical cell death in neurological disorders. J. Mol. Neurosci. 66, 197–206. doi: 10.1007/s12031-018-1155-6
Wyant, K. J., Ridder, A. J., and Dayalu, P. (2017). Huntington’s disease-update on treatments. Curr. Neurol. Neurosci. Rep. 17:33. doi: 10.1007/s11910-017-0739-9
Xie, Y., Hou, W., Song, X., Yu, Y., Huang, J., Sun, X., et al. (2016). Ferroptosis: process and function. Cell Death Differ. 23, 369–379. doi: 10.1038/cdd.2015.158
Xu, H., Perreau, V. M., Dent, K. A., Bush, A. I., Finkelstein, D. I., and Adlard, P. A. (2016). Iron regulates apolipoprotein E expression and secretion in neurons and astrocytes. J. Alzheimers Dis. 51, 471–487. doi: 10.3233/jad-150797
Xu, T., Ma, Y., Yuan, Q., Hu, H., Hu, X., Qian, Z., et al. (2020). Enhanced ferroptosis by oxygen-boosted phototherapy based on a 2-in-1 nanoplatform of ferrous hemoglobin for tumor synergistic therapy. ACS Nano 14, 3414–3425. doi: 10.1021/acsnano.9b09426
Yamaguchi, Y., Kasukabe, T., and Kumakura, S. (2018). Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis. Int. J. Oncol. 52, 1011–1022. doi: 10.3892/ijo.2018.4259
Yan, H. F., Tuo, Q. Z., Yin, Q. Z., and Lei, P. (2020). The pathological role of ferroptosis in ischemia/reperfusion-related injury. Zool. Res. 41, 220–230. doi: 10.24272/j.issn.2095-8137.2020.042
Yan, N., and Zhang, J. (2019). Iron metabolism, ferroptosis, and the links with Alzheimer’s disease. Front. Neurosci. 13:1443. doi: 10.3389/fnins.2019.01443
Yang, W. S., and Stockwell, B. R. (2008). Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 15, 234–245. doi: 10.1016/j.chembiol.2008.02.010
Yang, W. S., and Stockwell, B. R. (2016). Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 26, 165–176. doi: 10.1016/j.tcb.2015.10.014
Yang, W. S., SriRamaratnam, R., Welsch, M. E., Shimada, K., Skouta, R., Viswanathan, V. S., et al. (2014). Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331. doi: 10.1016/j.cell.2013.12.010
Ye, J., Jiang, X., Dong, Z., Hu, S., and Xiao, M. (2019). Low-Concentration PTX and rsl3 inhibits tumor cell growth synergistically by inducing ferroptosis in mutant p53 hypopharyngeal squamous carcinoma. Cancer Manag. Res. 11, 9783–9792. doi: 10.2147/cmar.s217944
Yu, H., Guo, P., Xie, X., Wang, Y., and Chen, G. (2017). Ferroptosis, a new form of cell death and its relationships with tumourous diseases. J. Cell. Mol. Med. 21, 648–657. doi: 10.1111/jcmm.13008
Yu, H., Yang, C., Jian, L., Guo, S., Chen, R., Li, K., et al. (2019). Sulfasalazineinduced ferroptosis in breast cancer cells is reduced by the inhibitory effect of estrogen receptor on the transferrin receptor. Oncol. Rep. 42, 826–838. doi: 10.3892/or.2019.7189
Yuan, H., Li, X., Zhang, X., Kang, R., and Tang, D. (2016). Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem. Biophys. Res. Commun. 478, 1338–1343. doi: 10.1016/j.bbrc.2016.08.124
Zhang, P., Chen, L., Zhao, Q., Du, X., Bi, M., Li, Y., et al. (2020). Ferroptosis was more initial in cell death caused by iron overload and its underlying mechanism in Parkinson’s disease. Free Radic. Biol. Med. 152, 227–234. doi: 10.1016/j.freeradbiomed.2020.03.015
Zhang, L., Liu, W., Liu, F., Wang, Q., Song, M., Yu, Q., et al. (2020). IMCA induces ferroptosis mediated by SLC7A11 through the AMPK/mTOR pathway in colorectal cancer. Oxid. Med. Cell. Longev. 2020:1675613. doi: 10.1155/2020/1675613
Zhang, Z., Yao, Z., Wang, L., Ding, H., Shao, J., Chen, A., et al. (2018). Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy 14, 2083–2103. doi: 10.1080/15548627.2018.1503146
Zhao, Z. (2019). Iron and oxidizing species in oxidative stress and Alzheimer’s disease. Aging Med. 2, 82–87. doi: 10.1002/agm2.12074
Keywords: ferroptosis, stroke, neurodegenerative disease, Alzheimer’s disease, Parkinson’s disease, iron
Citation: Lei J, Chen Z, Song S, Sheng C, Song S and Zhu J (2020) Insight Into the Role of Ferroptosis in Non-neoplastic Neurological Diseases. Front. Cell. Neurosci. 14:231. doi: 10.3389/fncel.2020.00231
Received: 29 April 2020; Accepted: 01 July 2020;
Published: 06 August 2020.
Edited by:
Anwen Shao, Zhejiang University, ChinaReviewed by:
Sheng Tu, Zhejiang University, ChinaLingfei Li, The University of Hong Kong, Hong Kong
Copyright © 2020 Lei, Chen, Song, Sheng, Song and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianming Zhu, zhujianming2006@163.com
 Jianwei Lei
Jianwei Lei  Jianming Zhu
Jianming Zhu