Chamazulene-Rich Artemisia arborescens Essential Oils Affect the Cell Growth of Human Melanoma Cells
Abstract
:1. Introduction
2. Results
2.1. Chemical Analysis
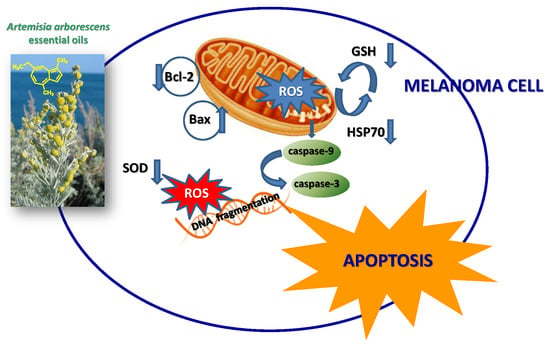
2.2. Biological Assays
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Isolation of Essential Oil
4.3. GC and GC-MS Analysis and Identification of Compounds
4.4. Identification of Compounds
4.5. Cell Culture and Treatments
4.6. MTT Bioassay
4.7. Lactate Dehydrogenase (LDH) Release
4.8. Activity of Caspase-3
4.9. DNA Analysis by COMET Assay
4.10. Western Blot Analysis
4.11. Reactive Oxygen Species Assay
4.12. Measurement of GSH levels
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vallès, J.; Garnatje, T. Artemisia and its allies: Genome organization and evolution and their biosystematic, taxonomic and phylogenetic implications in the Artemisiinae and related subtribes (Asteraceae, Anthemideae). Plant Genome Biodivers. Evol. 2005, 1, 255–285. [Google Scholar]
- Tutin, T.G.; Persson, K.; Gutermann, W. Compositae: Artemisia. In Flora Europaea; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: London, UK, 1976; pp. 178–186. [Google Scholar]
- Costa, R.; Ragusa, S.; Russo, M.; Certo, G.; Franchina, F.A.; Zanotto, A.; Grasso, E.; Mondello, L.; Germanò, M.P. Phytochemical screening of Artemisia arborescens L. by means of advanced chromatographic techniques for identification of health-promoting compounds. J. Pharm. Biomed. Anal. 2016, 117, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. Genus: A review of bioactive essential oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janaćkovića, P.; Rajčevića, N.; Gavrilovića, M.; Novakovića, J.; Giwelib, A.; Steševićc, D.; Marina, P.D. Essential oil composition of five Artemisia (Compositae) species in regards to chemophenetics. Biochem. Syst. Ecol. 2019, 87, 103960–103968. [Google Scholar] [CrossRef]
- Militello, M.; Carrubba, A.; Blázquez, M.A. Artemisia arborescens L.: Essential oil composition and effects of plant growth stage in some genotypes from Sicily. J. Essent. Oil Res. 2012, 24, 229–235. [Google Scholar] [CrossRef]
- Michelakis, E.C.; Evergetis, E.; Koulocheri, S.D.; Haroutounian, S.A. Exploitation of Artemisia arborescens as a renewable source of chamazulene: Seasonal variation and distillation conditions. Nat. Prod. Commun. 2016, 10, 1515–1519. [Google Scholar] [CrossRef] [Green Version]
- Ballero, M.; Poli, F.; Sacchetti, G.; Loi, M.C. Ethnobotanical research on the territory of Flumini maggiore (southwestern Sardinia). Fitoterapia 2001, 72, 788–801. [Google Scholar] [CrossRef]
- Lo Presti, M.; Crupi, M.L.; Zellner, B.d’A.; Dugo, G.; Mondello, L.; Dugo, P.; Ragusa, S. Characterization of Artemisia arborescens L. (Asteraceae) leaf-derived essential oil from Southern Italy. J. Essent. Oil Res. 2007, 19, 218–224. [Google Scholar] [CrossRef]
- Saddi, M.; Sanna, A.; Cottiglia, F.; Chisu, L.; Casu, L.; Bonsignore, L.; De Logu, A. Antiherpevirus activity of Artemisia arborescens essential oil and inhibition of lateral diffusion in Vero cells. Ann. Clin. Microbiol. Antimicrob. 2007, 26, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Militello, M.; Settanni, L.; Aleo, A.; Mammina, C.; Moschetti, G.; Giammanco, G.M.; Blàzquez, M.A.; Carrubba, A. Chemical composition and antibacterial potential of Artemisia arborescens L. essential oil. Curr. Microbiol. 2011, 62, 1274–1281. [Google Scholar] [CrossRef] [Green Version]
- Younes, K.; Merghache, S.; Djabou, N.; Merghache, D.; Muselli, A.; Tabti, B.; Costa, J. Chemical composition, antibacterial and antioxidant activities of a new essential oil chemotype of Algerian Artemisia arborescens L. Afr. J. Pharm. Pharm. 2012, 6, 2912–2921. [Google Scholar] [CrossRef] [Green Version]
- Ornano, L.; Venditti, A.; Ballero, M.; Sanna, C.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Papa, F.; Vittori, S.; Maggi, F.; et al. Chemopreventive and antioxidant activity of the chamazulene-rich essential oil obtained from Artemisia arborescens L. growing on the isle of La Maddalena, Sardinia, Italy. Chem. Biodivers. 2013, 10, 1464–1475. [Google Scholar] [CrossRef] [PubMed]
- Said, M.E.-A.; Militello, M.; Saia, S.; Settanni, L.; Aleo, A.; Mammina, C.; Bombarda, I.; Vanloot, P.; Roussel, C.; Dupuy, N. Artemisia arborescens essential oil composition, enantiomeric distribution, and antimicrobial activity from different wild populations from the Mediterranean area. Chem. Biodivers. 2016, 13, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Pappas, R.; Sheppard-Hanger, S. Artemisia arborescens essential oil of the Pacific Northwest: A high chamazulene, low thujone essential oil with potential skin-care applications. Aromather. J. 2000, 10, 30–33. [Google Scholar]
- Sacco, T.; Frattini, C.; Bicchi, C. Constituents of essential oil of Artemisia arborescens. Planta Med. 1983, 47, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Cardile, V.; Avola, R.; Graziano, A.C.E.; Piovano, M.; Russo, A. Cytotoxicity of demalonylthyrsiflorin A, a semisynthetic labdane-derived diterpenoid, to melanoma cells. Toxicol. Vitr. 2018, 47, 274–280. [Google Scholar] [CrossRef]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 26, 87. [Google Scholar] [CrossRef] [Green Version]
- Aguissa-Touré, A.H.; Li, G. Genetic alterations of PTEN in human melanoma. Cell. Mol. Life Sci. 2012, 69, 1475–1491. [Google Scholar] [CrossRef]
- Nogueira, V.; Hay, N. Molecular pathways: Reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin. Cancer Res. 2013, 19, 4309–4314. [Google Scholar] [CrossRef] [Green Version]
- Sznarkowska, A.; Kostecka, A.; Meller, K.; Bielawski, K. Inhibition of cancer antioxidant defense by natural compounds. Oncotarget 2017, 8, 15996–16016. [Google Scholar] [CrossRef] [Green Version]
- Lam, Y.; Ng, T.B.; Yao, R.M.; Shi, J.; Xu, K.; Wing Sze, S.C.; Zhang, K.Y. Evaluation of chemical constituents and important mechanism of pharmacological biology in Dendrobium Plants. Evid Based Complement. Altern. Med. 2015, 841752, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marongiu, B.; Piras, A.; Porcedda, S. Comparative analysis of the oil and supercritical CO2 extract of Artemisia arborescens L. and Helichrysum splendidum (Thunb.). Less. Nat. Prod. Res. 2010, 20, 421–428. [Google Scholar] [CrossRef]
- Riahi, L.; Chograni, H.; Masmoudi, A.S.; Cherif, A. Genetic resources of Tunisian Artemisia arborescens L. (Asteraceae), pattern of volatile metabolites concentration and bioactivity and implication for conservation. Biochem. Syst. Ecol. 2019, 87, 103952–103963. [Google Scholar] [CrossRef]
- Formisano, C.; Delfine, S.; Oliviero, F.; Tenore, G.C.; Rigano, D.; Senatore, F. Correlation among environmental factors, chemical composition and antioxidative properties of essential oil and extracts of chamomile (Matricaria chamomilla L.) collected in Molise (South-central Italy). Ind. Crop. Prod. 2015, 63, 256–263. [Google Scholar] [CrossRef]
- Appendino, G.; Gariboldi, P. The stereochemistry of matricin and 4-epimatricin, proazulene sesquiterpene lactones from Artemisia arborescens. Phytochemistry 1982, 21, 2555–2557. [Google Scholar] [CrossRef]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem. Toxicol. 2013, 55, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabro, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA A Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cote, H.; Boucher, M.A.; Pichette, A.; Legault, J. Anti-Inflammatory, antioxidant, antibiotic, and cytotoxic activities of Tanacetum vulgare L. essential oil and its constituents. Medicines 2017, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Formisano, C.; Rigano, D.; Cardile, V.; Arnold, N.A.; Senatore, F. Comparative phytochemical profile and antiproliferative activity on human melanoma cells of essential oils of three Lebanese Salvia species. Ind. Crops Prod. 2016, 83, 492–499. [Google Scholar] [CrossRef]
- Chen, W.Q.; Xu, B.; Mao, J.W.; Wei, F.X.; Li, M.; Liu, T.; Jin, X.B.; Zhang, L.R. Inhibitory effects of α-pinene on hepatoma carcinoma cell proliferation. Asian Pac. J. Cancer Prev. 2014, 7, 3293–3297. [Google Scholar] [CrossRef] [Green Version]
- Girola, N.; Figueiredo, C.R.; Farias, C.F.; Azevedo, R.A.; Ferreira, A.K.; Teixeira, S.F.; Capello, T.M.; Martins, E.G.; Matsuo, A.L.; Travassos, L.R.; et al. Camphene isolated from essential oil of Piper cernuum (Piperaceae) induces intrinsic apoptosis in melanoma cells and displays antitumor activity in vivo. Biochem. Biophys. Res. Commun. 2015, 467, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Goloudina, A.R.; Demidov, O.N.; Garrido, C. Inhibition of HSP70: A challenging anti-cancer strategy. Cancer Lett. 2012, 325, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Budina-Kolomets, A.; Webster, M.R.; Leu, J.I.; Jennis, M.C.; Krepler, C.; Guerrini, A.; Kossenkov, A.V.; Xu, W.; Karakousis, G.C.; Schuchter, L.M.; et al. HSP70 Inhibition Limits FAK-Dependent Invasion and Enhances the Response to Melanoma Treatment with BRAF Inhibitors. Cancer Res. 2016, 76, 2720–2730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, M.E. The HSP70 family and cancer. Carcinogenesis 2013, 34, 1181–1188. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Yu, G.; Shen, Y. The naturally occurring xanthone α-mangostin induces ROS mediated cytotoxicity in non-small scale lung cancer cells. Saudi J. Biol. Sci. 2018, 25, 1090–1095. [Google Scholar] [CrossRef]
- Ikwegbue, P.C.; Masamba, P.; Oyinloye, B.E.; Kappo, A.P. Roles of heat shock proteins in apoptosis, oxidative Stress, human inflammatory diseases, and cancer. Pharmaceuticals 2018, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Montero, A.J.; Jassem, J. Cellular redox pathways as a therapeutic target in the treatment of cancer. Drugs 2011, 71, 1385–1396. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress and cancer: Have we moved forward? Biochem. J. 2007, 401, 1–11. [Google Scholar] [CrossRef]
- Kim, B.; Srivastava, S.K.; Kim, S.H. Caspase-9 as a therapeutic target for treating cancer. Expert Opin. Targets 2017, 19, 113–127. [Google Scholar] [CrossRef]
- Formisano, C.; Oliviero, F.; Rigano, D.; Saab, A.M.; Senatore, F. Chemical composition of essential oils and in vitro antioxidant properties of extracts and essential oils of Calamintha origanifolia and Micromeria myrtifolia, two Lamiaceae from the Lebanon flora. Ind. Crop. Prod. 2014, 62, 405–411. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]









| No | Place of Collection | Coordinate | Altitude s/l | Date | Essential Oil Yield % |
|---|---|---|---|---|---|
| F1 | Capo Zafferano, Sicily | 38°06′38” N; 13°31′47” E | 22 m | 13 June 2018 | 1.25 ± 0.04 |
| L1 | Capo Zafferano, Sicily | 38°06′38” N; 13°31′47” E | 22 m | 13 June 2018 | 1.32 ± 0.03 |
| F2 | Alimena, Sicily | 37°40′50” N; 14°05′01” E | 645 m | 19 June 2018 | 0.93 ± 0.03 |
| L2 | Alimena, Sicily | 37°40′50” N; 14°05′01” E | 645 m | 19 June 2018 | 0.20 ± 0.02 |
| F3 | Lami, Lipari Island | 38°29′55” N, 14°56′47” E | 282 m | 16 June 2018 | 1.08 ± 0.03 |
| L3 | Lami, Lipari Island | 38°29′55” N, 14°56′47” E | 282 m | 16 June 2018 | 1.53 ± 0.04 |
| F4 | Acqua Calda, Lipari Island | 38°31′11” N, 14°56′00” E | 2 m | 17 June 2018 | 1.11 ± 0.03 |
| L4 | Acqua Calda, Lipari Island | 38°31′11” N, 14°56′00” E | 2 m | 17 June 2018 | 1.12 ± 0.03 |
| Ki a | Ki b | Compound | F1 % d | F2 % d | F3 % d | F4 % d | L1 % d | L2 % d | L3 % d | L4 % d | Id. c |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Monoterpenes | 7.4 | 2.6 | 5.9 | 5.2 | 6.2 | 0.9 | 9.9 | 0.5 | |||
| 925 | 1014 | Tricyclene | t | 1, 2 | |||||||
| 930 | 1014 | α-Thujene | 0.2 | t | t | 0.1 | 1,2 | ||||
| 938 | 1032 | α-Pinene | 0.7 | 0.2 | 1.9 | 2.3 | 0.6 | 3.7 | 0.2 | 1, 2, 3 | |
| 938 | 1117 | Verbenene | t | 1, 2 | |||||||
| 953 | 1076 | Camphene | 0.7 | 2.6 | 2.7 | 0.7 | 3.7 | 0.2 | 1, 2 | ||
| 973 | 1132 | Sabinene | 1.2 | 0.8 | 1.2 | 0.7 | 1, 2 | ||||
| 993 | 1174 | Myrcene | 1.5 | 0.4 | 1.1 | 0.1 | 1, 2, 3 | ||||
| 1005 | 1150 | α-Phellandrene | 0.1 | 1, 2 | |||||||
| 1012 | 1157 | δ3-Carene | 0.2 | 0.3 | 0.1 | ||||||
| 1013 | 1189 | α-Terpinene | 0.7 | 0.4 | t | 0.6 | 0.1 | t | 1, 2, 3 | ||
| 1025 | 1278 | p-Cymene | 0.2 | 0.1 | 0.3 | t | 1, 2, 3 | ||||
| 1029 | 1218 | β-Phellandrene | 0.4 | t | 1, 2 | ||||||
| 1030 | 1203 | Limonene | 0.3 | 1, 2, 3 | |||||||
| 1049 | 1265 | (E)-β-Ocimene | 2.2 | ||||||||
| 1057 | 1256 | γ-Terpinene | 1.4 | 0.6 | 0.1 | 1.1 | t | 1, 2, 3 | |||
| 1086 | 1265 | Terpinolene | 0.3 | 0.1 | 1.3 | 0.2 | 1, 2, 3 | ||||
| Oxygenated monoterpenes | 42.5 | 48.5 | 58.3 | 55.6 | 31.5 | 37.9 | 71.8 | 24.3 | |||
| 1034 | 1213 | 1,8-Cineole | 0.2 | 0.2 | 0.1 | 1, 2, 3 | |||||
| 1063 | 1555 | (Z)-Sabinene hydrate | 0.8 | 0.1 | 0.8 | 1, 2 | |||||
| 1098 | 1457 | (E)-Sabinene hydrate | 0.2 | 1, 2 | |||||||
| 1098 | 1553 | Linalool | 1.2 | 1, 2, 3 | |||||||
| 1105 | 1431 | α-Thujone | 0.8 | 1.3 | 0.6 | 1, 2 | |||||
| 1115 | 1451 | β-Thujone | 26.7 | 44.8 | 13.5 | 34.4 | 1, 2 | ||||
| 1125 | 1540 | Chrysanthenone | 0.1 | 1, 2 | |||||||
| 1125 | 1623 | Myrcenol | 0.2 | 1, 2 | |||||||
| 1126 | 1637 | cis-p-Menth-2-en-1-ol | 0.1 | 1, 2 | |||||||
| 1145 | 1532 | Camphor | 8.5 | 58.2 | 54.2 | 10.0 | 65.6 | 21.5 | 1, 2, 3 | ||
| 1167 | 1718 | Borneol | 0.2 | t | 0.2 | 1.7 | 0.2 | 1, 2, 3 | |||
| 1176 | 1611 | Terpinen-4-ol | 2.2 | 1.4 | 1.4 | 2.4 | 0.6 | 4.5 | 2.5 | 1, 2, 3 | |
| 1189 | 1706 | α-Terpineol | 0.3 | 0.2 | 0.3 | 0.1 | 1, 2, 3 | ||||
| 1192 | 1805 | Myrtenol | t | 1, 2 | |||||||
| 1233 | 1662 | Pulegone | 1.3 | 1, 2 | |||||||
| 1259 | 1665 | Linalyl acetate | 0.1 | ||||||||
| 1259 | 1891 | Myrtanol | 0.5 | 0.3 | 1, 2 | ||||||
| 1261 | 1809 | Perilla aldehyde | 0.4 | 0.2 | 0.2 | 1, 2 | |||||
| 1284 | 1597 | Bornyl acetate | 1.6 | 1.9 | 1, 2, 3 | ||||||
| 1289 | 1812 | p-Mentha-1,3-dien-7-al (α-Terpinen-7-al) | 0.1 | 1, 2 | |||||||
| 1329 | 1949 | Piperitenone | 1.0 | 1, 2 | |||||||
| 1602 | 1893 | Geranyl isovalerate | 0.2 | 0.3 | 1, 2 | ||||||
| Sesquiterpenes | 5.6 | 2.2 | - | - | 4.8 | 1.5 | 1.7 | 3.2 | |||
| 1373 | 1493 | α-Ylangene | 0.3 | 1, 2 | |||||||
| 1377 | 1497 | α-Copaene | 0.3 | 0.2 | 0.1 | 1, 2 | |||||
| 1385 | 1535 | β-Bourbonene | 0.1 | 0.1 | 1, 2 | ||||||
| 1418 | 1612 | (E)-Caryophyllene | 0.8 | 0.3 | 1.2 | 1.7 | 1.3 | 1, 2, 3 | |||
| 1455 | 1689 | α-Humulene | 0.1 | 0.1 | 0.2 | 1, 2 | |||||
| 1463 | 1667 | allo-Aromadendrene | 0.1 | ||||||||
| 1477 | 1726 | Germacrene D | 2.6 | 1.7 | 2.4 | 1.5 | 1.1 | 1, 2 | |||
| 1491 | 1756 | Bicyclogermacrene | 0.2 | 0.1 | 1, 2 | ||||||
| 1498 | 1744 | α-Selinene | 0.2 | ||||||||
| 1503 | 1740 | α-Muurolene | 0.1 | 1, 2 | |||||||
| 1506 | 1760 | (E,E)-α-Farnesene | 0.4 | 0.2 | 1, 2 | ||||||
| 1509 | 1746 | cis-(Z)-α-Bisabolene | 0.2 | t | 1,2 | ||||||
| 1510 | 1743 | β-Bisabolene | 0.3 | 1, 2 | |||||||
| 1526 | 1773 | δ-Cadinene | 0.2 | 0.2 | 0.1 | 1, 2 | |||||
| 1541 | 1918 | α-Calacorene | 0.4 | 0.2 | 1, 2 | ||||||
| 1554 | 1856 | Germacrene B | t | ||||||||
| Oxygenated sesquiterpenes | 1.6 | - | - | - | 2.4 | - | - | 4.0 | |||
| 1578 | 2150 | Spathulenol | 0.2 | 0.1 | 1, 2 | ||||||
| 1579 | 2208 | Caryophyllene oxide | 0.1 | 0.4 | 0.8 | 1, 2, 3 | |||||
| 1605 | (2R,5E)-Caryophyll-5-en-12-al | t | 1, 2 | ||||||||
| 1627 | (2S,5E)-Caryophyll-5-en-12-al | t | 0.5 | 1, 2 | |||||||
| 1632 | 2371 | Caryophylla-3,8(13)-dien-5α-ol | 0.4 | 0.7 | 0.4 | 1, 2 | |||||
| 1640 | 2361 | Caryophylla-4(12),8(13)-dien-5β-ol | 0.3 | 0.5 | 1, 2 | ||||||
| 1645 | 2209 | Torreyol | 0.4 | ||||||||
| 1650 | 2258 | β-Eudesmol | 0.3 | 0.5 | 1.3 | 1, 2 | |||||
| 1652 | 2255 | α-Cadinol | 0.4 | 0.3 | 1, 2 | ||||||
| 1665 | 2371 | Caryophylla-3,8(13)-dien-5β-ol | 0.3 | 1, 2 | |||||||
| 1765 | 2518 | cis-Lanceol | 0.1 | 1, 2 | |||||||
| 2075 | Caryophylla-2(12),6(13)-dien-5-one | t | 1, 2 | ||||||||
| Carbonilic compounds | 0.5 | 0.7 | - | - | 0.2 | 0.2 | - | - | |||
| 963 | 1543 | Benzaldehyde | t | 1, 2, 3 | |||||||
| 989 | 1349 | 6-Methyl-5-hepten-2-one | 0.5 | 0.4 | 0.1 | 1, 2 | |||||
| 1621 | 1(2H)-Naphthalenone, 2,2,3-trimethyl | 0.1 | 0.2 | 1, 2 | |||||||
| 1702 | 2416 | 4-Isopropyl-6-methyl-1-tetralone | 0.3 | 1, 2 | |||||||
| Phenols | 0.6 | 0.2 | - | - | - | - | - | - | |||
| 1206 | 1805 | 4-Methyl veratrole | 0.5 | 0.2 | 1, 2 | ||||||
| 1400 | 2016 | Methyl eugenol | 0.1 | 1, 2, 3 | |||||||
| Hydrocarbons | 38.9 | 37.4 | 34.2 | 39.0 | 51.8 | 55.6 | 14.9 | 63.0 | |||
| 1338 | Naphthalene, 1,4-dihydro-2,5,8-trimethyl- | 0.1 | 1, 2 | ||||||||
| 1358 | Naphthalene, 1,2-dihydro-2,5,8-trimethyl | 0.1 | 0.2 | 1, 2 | |||||||
| 1735 | 2434 | Chamazulene | 38.8 | 37.4 | 34.2 | 39.0 | 51.7 | 55.4 | 14.9 | 63.0 | 1, 2 |
| Others | 0.2 | 0.1 | - | - | 0.3 | - | - | - | |||
| 1005 | 1174 | 2-Methylbutyl isobutanoate | 0.1 | 1, 2 | |||||||
| 1398 | 1904 | Benzyl isovalerate | 0.2 | 1, 2 | |||||||
| 1189 | 1789 | Methyl salicylate | 0.2 | 1, 2, 3 | |||||||
| 1233 | 1484 | cis-3-Hexenyl-2-Methylbutanoate | 0.1 | 1, 2 | |||||||
| TOTAL | 97.3 | 91.7 | 98.4 | 99.8 | 97.2 | 96.1 | 98.3 | 95.0 |
| Treatments | A375 Cells |
|---|---|
| Essential Oils | IC50 a (µg/mL) |
| F1 | 21.1 ± 0.17 |
| L1 | 23.4 ± 0.15 |
| F2 | 15.3 ± 0.13 |
| L2 | 16.2 ± 0.17 |
| F3 | 16.3 ± 0.14 |
| L3 | 19.7 ± 0.18 |
| F4 | 6.7 ± 0.22 |
| L4 | 4.5 ± 0.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, A.; Bruno, M.; Avola, R.; Cardile, V.; Rigano, D. Chamazulene-Rich Artemisia arborescens Essential Oils Affect the Cell Growth of Human Melanoma Cells. Plants 2020, 9, 1000. https://doi.org/10.3390/plants9081000
Russo A, Bruno M, Avola R, Cardile V, Rigano D. Chamazulene-Rich Artemisia arborescens Essential Oils Affect the Cell Growth of Human Melanoma Cells. Plants. 2020; 9(8):1000. https://doi.org/10.3390/plants9081000
Chicago/Turabian StyleRusso, Alessandra, Maurizio Bruno, Rosanna Avola, Venera Cardile, and Daniela Rigano. 2020. "Chamazulene-Rich Artemisia arborescens Essential Oils Affect the Cell Growth of Human Melanoma Cells" Plants 9, no. 8: 1000. https://doi.org/10.3390/plants9081000