Optimization of Anodization Parameters in Ti-30Ta Alloy
Abstract
:1. Introduction
2. Materials and Methods
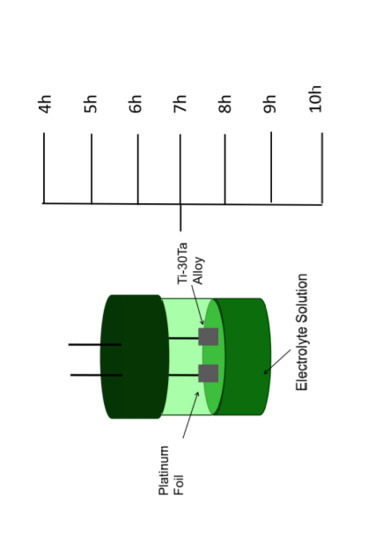
2.1. Process of Ti-30Ta Alloy
2.2. Surface Treatment
2.3. Characterization of Substrates
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Verma, R.P. Materials Today: Proceedings Titanium based biomaterial for bone implants: A mini review. Mater. Today Proc. 2020, 26, 3148–3151. [Google Scholar] [CrossRef]
- Biswal, T.; BadJena, S.K.; Pradhan, D. Sustainable biomaterials and their applications: A short review. Mater. Today Proc. 2020. Available online: https://www.sciencedirect.com/science/article/pii/S2214785320305423 (accessed on 24 January 2020). [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Pandey, A.; Awasthi, A.; Saxena, K.K. Metallic implants with properties and latest production techniques: A review. Adv. Mater. Process. Technol. 2020, 6, 405–440. [Google Scholar] [CrossRef]
- Delanois, R.E.; Mistry, J.B.; Gwam, C.U.; Mohamed, N.S.; Choksi, U.S.; Mont, M.A. Current Epidemiology of Revision Total Knee Arthroplasty in the United States. J. Arthroplasty 2017, 32, 2663–2668. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Recent Progress in Research and Development of Metallic Structural Biomaterials with Mainly Focusing on Mechanical Biocompatibility. Mater. Trans. 2018, 59, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kurella, A.; Dahotre, N.B. Review paper: Surface modification for bioimplants: The role of laser surface engineering. J. Biomater. Appl. 2005, 20, 5–50. [Google Scholar] [CrossRef] [PubMed]
- Grimes, C.A.; Varghese, O.K.; Feng, X.; Mor, G.K.; LaTempa, T.J.; Basham, J.I. Ta3N5 Nanotube Arrays for Visible Light Water Photoelectrolysis. Nano Lett. 2010, 10, 948–952. [Google Scholar] [CrossRef]
- Walker, P.R.; LeBlanc, J.; Sikorska, M. Effects of aluminum and other cations on the structure of brain and liver chromatin. Biochemistry 1989, 28, 3911–3915. [Google Scholar] [CrossRef]
- Zaffe, D.; Bertoldi, C.; Consolo, U. Accumulation of aluminium in lamellar bone after implantation of titanium plates, Ti-6Al-4V screws, hydroxyapatite granules. Biomaterials 2004, 25, 3837–3844. [Google Scholar] [CrossRef]
- Besinis, A.; Hadi, S.D.; Le, H.R.; Tredwin, C.; Handy, R.D. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotoxicology 2017, 11, 327–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J. Metallic biomaterials: State of the art and new challenges. Fundam. Biomater. Met. 2018, 1–33. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Reports 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Niinomi, M. Ti-25Ta alloy with the best mechanical compatibility in Ti-Ta alloys for biomedical applications. Mater. Sci. Eng. C 2009, 29, 1061–1065. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Niinomi, M.; Akahori, T. Effects of Ta content on Young’s modulus and tensile properties of binary Ti-Ta alloys for biomedical applications. Mater. Sci. Eng. A 2004, 371, 283–290. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Niinomi, M.; Akahori, T.; Fukui, H.; Toda, H. Corrosion resistance and biocompatibility of Ti-Ta alloys for biomedical applications. Mater. Sci. Eng. A 2005, 398, 28–36. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Niinomi, M.; Akahori, T. Decomposition of martensite α″ during aging treatments and resulting mechanical properties of Ti-Ta alloys. Mater. Sci. Eng. A 2004, 384, 92–101. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Niinomi, M. Microstructures and mechanical properties of Ti-50 mass% Ta alloy for biomedical applications. J. Alloys Compd. 2008, 466, 535–542. [Google Scholar] [CrossRef]
- Capellato, P.; Escada, A.L.A.; Popat, K.C.; Claro, A.P.R.A. Interaction between mesenchymal stem cells and Ti-30Ta alloy after surface treatment. J. Biomed. Mater. Res. Part A 2014, 102. [Google Scholar] [CrossRef]
- Capellato, P.; Smith, B.S.; Popat, K.C.; Alves Claro, A.P.R. Cellular functionality on nanotubes of Ti-30Ta alloy. In Materials Science Forum; Trans Tech Publications Ltd.: Foz de Iguaçu, Brazil, 2015; Volume 805, ISBN 9783038352365. [Google Scholar]
- Wong, J.Y.; Leach, J.B.; Brown, X.Q. Brown. Balance of chemistry, topography, and mechanics at the cell—biomaterial interface: Issues and challenges for assessing the role of substrate mechanics on cell response. Surf. Sci. 2004, 570, 119–133. [Google Scholar] [CrossRef]
- Capellato, P.; Riedel, N.A.; Williams, J.D.; Machado, J.P.B.; Popat, K.C.; Claro, A.P.R.A. Surface Modification on Ti-30Ta Alloy for Biomedical Application. Engineering 2013, 5, 707–713. [Google Scholar] [CrossRef] [Green Version]
- Capellato, P.; Riedel, N.A.; Williams, J.D.; Machado, J.P.B.; Ketul Popat, K.C.; Alves Claro, A.P.R. Ion Bean Etching on Ti-30Ta Alloy for Biomedical Application. In Materials Science Forum; Trans Tech Publications Ltd.: Foz de Iguaçu, Brazil, 2015; Volume 805, ISBN 9783038352365. [Google Scholar]
- Oh, S.; Daraio, C.; Chen, L.H.; Pisanic, T.R.; Fiñones, R.R.; Jin, S. Significantly accelerated osteoblast cell growth on aligned TiO2 nanotubes. J. Biomed. Mater. Res. Part A 2006, 78, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Simi, V.S.; Rajendran, N. Influence of tunable diameter on the electrochemical behavior and antibacterial activity of titania nanotube arrays for biomedical applications. Mater. Charact. 2017, 129, 67–79. [Google Scholar] [CrossRef]
- Regonini, D.; Bowen, C.R.; Jaroenworaluck, A.; Stevens, R. A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater. Sci. Eng. R Rep. 2013, 74, 377–406. [Google Scholar] [CrossRef] [Green Version]
- Capellato, P.; Smith, B.S.; Popat, K.C.; Claro, A.P.R.A. Fibroblast functionality on novel Ti30Ta nanotube array. Mater. Sci. Eng. C 2012, 32, 2060–2067. [Google Scholar] [CrossRef]
- Kim, I.-H.; Kwon, T.-Y.; Kim, K.-H. Wetting Behavior of Dental Implants. Wetting Wettability 2015. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Rath, B.; Tingart, M.; Eschweiler, J. Role of implants surface modification in osseointegration: A systematic review. J. Biomed. Mater. Res. Part A 2020, 108, 470–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marien, C.B.D.; Cottineau, T.; Robert, D.; Drogui, P. TiO2 Nanotube arrays: Influence of tube length on the photocatalytic degradation of Paraquat. Appl. Catal. B Environ. 2016, 194, 1–6. [Google Scholar] [CrossRef]
- Aguirre Ocampo, R.; Echeverría Echeverría, F. Effect of the anodization parameters on TiO2 nanotubes characteristics produced in aqueous electrolytes with CMC. Appl. Surf. Sci. 2019, 469, 994–1006. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Schmuki, P. Less known facts and findings about TiO2 nanotubes. Nanoscale 2020, 12, 8119–8132. [Google Scholar] [CrossRef]
- Smith, B.S.; Yoriya, S.; Grissom, L.; Grimes, C.A.; Popat, K.C. Hemocompatibility of titania nanotube arrays. J. Biomed. Mater. Res. Part A 2010, 95, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.S.; Capellato, P.; Kelley, S.; Gonzalez-Juarrero, M.; Popat, K.C. Reduced in vitro immune response on titania nanotube arrays compared to titanium surface. Biomater. Sci. 2013, 1. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.S.; Yoriya, S.; Johnson, T.; Popat, K.C. Dermal fibroblast and epidermal keratinocyte functionality on titania nanotube arrays. Acta Biomater. 2011, 7, 2686–2696. [Google Scholar] [CrossRef]
- Hu, N.; Wu, Y.; Xie, L.; Yusuf, S.M.; Gao, N.; Starink, M.J.; Tong, L.; Chu, P.K.; Wang, H. Enhanced interfacial adhesion and osseointegration of anodic TiO2 nanotube arrays on ultra-fine-grained titanium and underlying mechanisms. Acta Biomater. 2020, 106, 360–375. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Lin, J.; Biesiekierski, A.; Li, Y.; Wen, C. Effect of Anodized TiO2-Nb2O5-ZrO2 Nanotubes with Different Nanoscale Dimensions on the Biocompatibility of a Ti35Zr28Nb Alloy. ACS Appl. Mater. Interfaces 2020, 12, 6776–6787. [Google Scholar] [CrossRef] [PubMed]
- Sankara Narayanan, T.S.N.; Kim, J.; Park, H.W. Fabrication and synthesis of uniform TiO2 nanoporous and nanotubular structures on dual-phase Ti–6Al–4V alloy using electron-beam irradiation. Mater. Chem. Phys. 2020, 242, 122549. [Google Scholar] [CrossRef]
- El-Sayed, H.A.; Birss, V.I. Controlled growth and monitoring of tantalum oxide nanostructures. Nanoscale 2010, 2, 793–798. [Google Scholar] [CrossRef]
- El-sayed, H.A.; Birss, V.I. Controlled formation of Ta dimples or Ta oxide nanotubes by anodization. Nano Lett. 2006, 6, 2995–2999. [Google Scholar] [CrossRef]
- El-Sayed, H.; Singh, S.; Kruse, P. Formation of Dimpled Tantalum Surfaces from Electropolishing. J. Electrochem. Soc. 2007, 154, C728. [Google Scholar] [CrossRef]
- El-Sayed, H.A.; Birss, V.I. Controlled interconversion of nanoarray of Ta dimples and high aspect ratio Ta oxide nanotubes. Nano Lett. 2009, 9, 1350–1355. [Google Scholar] [CrossRef]
- Min, X.; Bai, P.; Emura, S.; Ji, X.; Cheng, C.; Jiang, B.; Tsuchiya, K. Effect of oxygen content on deformation mode and corrosion behavior in β-type Ti-Mo alloy. Mater. Sci. Eng. A 2017, 684, 534–541. [Google Scholar] [CrossRef]
- Fomby, P.; Cherlin, A.J.; Hadjizadeh, A.; Doillon, C.J.; Sueblinvong, V.; Weiss, D.J.; Bates, J.H.T.; Gilbert, T.; Liles, W.C.; Lutzko, C.; et al. Anodic oxide nanotube layers on Ti–Ta alloys: Substrate composition, microstructure and self-organization on two-size scales. Corros. Sci. 2006, 12, 181–204. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Akaki, T.; Nakata, J.; Terada, D.; Tsuji, N.; Koizumi, Y.; Minamino, Y.; Schmuki, P.; Fujimoto, S. Anodic oxide nanotube layers on Ti–Ta alloys: Substrate composition, microstructure and self-organization on two-size scales. Corros. Sci. 2009, 51, 1528–1533. [Google Scholar] [CrossRef]
- Porter, J.R.; Henson, A.; Ryan, S.; Popat, K.C. Biocompatibility and Mesenchymal Stem Cell Response to Poly (ε-Caprolactone) Nanowire Surfaces for Orthopedic Tissue Engineering. Tissue Eng. Part A 2009, 15, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Mat-Baharin, N.H.; Razali, M.; Mohd-Said, S.; Syarif, J.; Muchtar, A. Influence of alloying elements on cellular response and in-vitro corrosion behavior of titanium-molybdenum-chromium alloys for implant materials. J. Prosthodont. Res. 2020. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Mitri, F.F.; Ingle, A.P. The Bone Biology and the Nanotechnology for Bone Engineering and Bone Diseases. In Nanotechnology in Skin, Soft Tissue, and Bone Infections; Springer International Publishing: Amravati, India, 2020; pp. 223–244. [Google Scholar]
- Lorenzetti, M.; Kulkarni, C.V.; Iglič, A.; Patil-Sen, Y.; Junkar, I.; Kulkarni, M. Wettability studies of topologically distinct titanium surfaces. Colloids Surf. B 2015, 129, 47–53. [Google Scholar] [CrossRef]
- Capellato, P.; Claro, A.P.R.A.; Silva, G.; Zavaglia, C.A.C. Antimicrobial effect of TiO2 nanotubes coating for dental implant. Dent. Mater. 2018, 34, e21. [Google Scholar] [CrossRef]
- Fan, H.; Guo, Z. Bioinspired surfaces with wettability: Biomolecule adhesion behaviors. Biomater. Sci. 2020, 8, 1502–1535. [Google Scholar] [CrossRef]
- Popa, A.M.; Giazzon, M.; Davidson, P.; Ploux, L.; Liley, M.; Anselme, K. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef]
- Capellato, P.; Sachs, D.; Claro, A.P.R.A.; Silva, G.; Zavaglia, C.A.C. Comparative research of bacteria gram-negative and positive on ti-30ta alloy. Dent. Mater. 2019, 35, e6–e7. [Google Scholar] [CrossRef]






| Anodization | Nanotube Diameter | Nanotube Length |
|---|---|---|
| Time (h) | (nm) | (µm) |
| 4 | 26.41 ± 0.94 | 7.13 ± 1.57 |
| 5 | 00.00 | 00.00 |
| 6 | 28.79 ± 1.17 | 6.88 ± 1.73 |
| 7 | 32.67 ± 2.51 | 6.87 ± 1.68 |
| 8 | 42.77 ± 1.89 | 7.25 ± 0.97 |
| 9 | 57.21 ± 3.24 | 6.54 ± 2.09 |
| 10 | 42.32 ± 2.31 | 8.61 ± 1.36 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capellato, P.; Sachs, D.; Vasconcelos, L.V.B.; Melo, M.M.; Silva, G.; Ranieri, M.G.A.; Zavaglia, C.A.d.C.; Nakazato, R.Z.; Claro, A.P.R.A. Optimization of Anodization Parameters in Ti-30Ta Alloy. Metals 2020, 10, 1059. https://doi.org/10.3390/met10081059
Capellato P, Sachs D, Vasconcelos LVB, Melo MM, Silva G, Ranieri MGA, Zavaglia CAdC, Nakazato RZ, Claro APRA. Optimization of Anodization Parameters in Ti-30Ta Alloy. Metals. 2020; 10(8):1059. https://doi.org/10.3390/met10081059
Chicago/Turabian StyleCapellato, Patricia, Daniela Sachs, Lucas V. B. Vasconcelos, Miriam M. Melo, Gilbert Silva, Maria G. A. Ranieri, Cecilia A. de C. Zavaglia, Roberto Z. Nakazato, and Ana P. R. Alves Claro. 2020. "Optimization of Anodization Parameters in Ti-30Ta Alloy" Metals 10, no. 8: 1059. https://doi.org/10.3390/met10081059