Filtration Scheduling: Quality Changes in Freshly Produced Virgin Olive Oil
Abstract
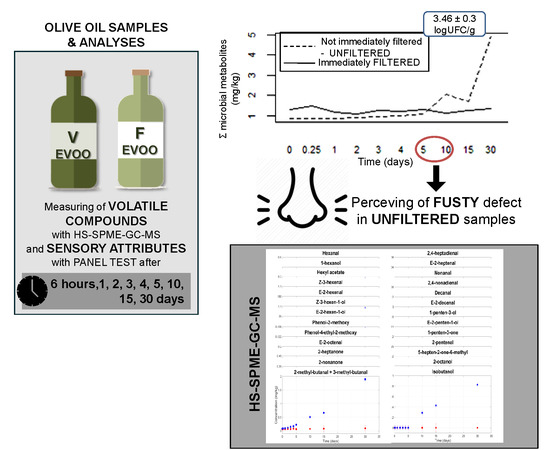
:1. Introduction
2. Materials and Methods
2.1. Olive Oil Samples
2.2. Chemicals and Reagents
2.3. Chemical Analyses
2.4. Turbidity Grade
2.5. Water Content and Water Activity
2.6. Solid Particles Content
2.7. Microbial Analyses
2.8. Sensory Analyses
2.9. Statistical Analyses
3. Results and Discussion
3.1. Turbidity Characterisation, Microbial Contamination, and Legal Requirements
3.2. Sensory Attributes
3.3. Volatile Compound Contents
3.3.1. Pleasant LOX Pathway Volatile Compound Contents
3.3.2. Unpleasant Volatile Compound Contents
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quiles, J.L.; Ramírez-Tortosa, M.C.; Yaqoob, P. (Eds.) Olive Oil and Health; CABI: Wallingford, UK, 2006. [Google Scholar]
- Morales, M.T.; Luna, G.; Aparicio, R. Comparative study of virgin olive oil sensory defects. Food Chem. 2005, 91, 293–301. [Google Scholar] [CrossRef]
- Zanoni, B. Which processing markers are recommended for measuring and monitoring the transformation pathways of main components of olive oil? Ital. J. Food Sci. 2014, 26, 3–11. [Google Scholar]
- Del Giudice, T.; Cavallo, C.; Caracciolo, F.; Cicia, G. What attributes of extra virgin olive oil are really important for consumers: A meta-analysis of consumers’ stated preferences. Agric. Food Econ. 2015, 3, 20. [Google Scholar] [CrossRef] [Green Version]
- European Union Commission. Regulation EEC 2568/91 on the characteristics of olive oil and olive pomace and their analytical methods. Off. J. Euro. Comm. L 1991, 248, 1–83. [Google Scholar]
- European Union Commission. Regulation EEC 2095/2016 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Euro. Comm. L 2016, 326, 1–6. [Google Scholar]
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G. Volatile compounds in virgin olive oil: Occurrence and their relationship with the quality. J. Chromatogr. A 2004, 1054, 17–31. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R., Jr.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Trapani, S.; Migliorini, M.; Cherubini, C.; Cecchi, L.; Canuti, V.; Fia, G.; Zanoni, B. Direct quantitative indices for ripening of olive oil fruits to predict harvest time. Eur. J. Lipid Sci. Technol. 2016, 118, 1202–1212. [Google Scholar] [CrossRef]
- Trapani, S.; Breschi, C.; Cecchi, L.; Guerrini, L.; Mulinacci, N.; Parenti, A.; Canuti, V.; Picchi, M.; Caruso, G.; Gucci, R.; et al. Indirect indices of oxidative damage to phenolic compounds for the implementation of olive paste malaxation optimization charts. J. Food Eng. 2017, 207, 24–34. [Google Scholar] [CrossRef]
- Guerrini, L.; Zanoni, B.; Breschi, C.; Angeloni, G.; Masella, P.; Calamai, L.; Parenti, A. Understanding olive oil stability using filtration and high hydrostatic pressure. Molecules 2020, 25, 420. [Google Scholar] [CrossRef] [Green Version]
- Aparicio, R.; Morales, M.T.; Alonso, M.V. Relationship between volatile compounds and sensory attributes of olive oils by the sensory wheel. J. Am. Oil Chem. Soc. 1996, 73, 1253–1264. [Google Scholar] [CrossRef]
- Campestre, C.; Angelini, G.; Gasbarri, C.; Angerosa, F. The compounds responsible for the sensory profile in monovarietal virgin olive oils. Molecules 2017, 22, 1833. [Google Scholar] [CrossRef] [PubMed]
- Guth, H.; Grosch, W. A comparative study of the potent odorants of different virgin olive oils. Lipid/Fett 1991, 93, 335–339. [Google Scholar] [CrossRef]
- Lerma-García, M.J.; Simó-Alfonso, E.F.; Bendini, A.; Cerretani, L. Metal oxide semiconductor sensors for monitoring of oxidative status evolution and sensory analysis of virgin olive oils with different phenolic content. Food Chem. 2009, 117, 608–614. [Google Scholar] [CrossRef]
- Cayuela, J.A.; Gómez-Coca, R.B.; Moreda, W.; Pérez-Camino, M.C. Sensory defects of virgin olive oil from a microbiological perspective. Trends Food Sci. Technol. 2015, 43, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Angerosa, F.; Lanza, B.; Marsilio, V. Biogenesis of «fusty» defect in virgin olive oils. Grasas Aceites 1996, 47. [Google Scholar] [CrossRef] [Green Version]
- Aparicio, R.; Morales, M.T.; García-González, D.L. Towards new analyses of aroma and volatiles to understand sensory perception of olive oil. Eur. J. Lipid Sci. Technol. 2012, 114, 1114–1125. [Google Scholar] [CrossRef]
- Guerrini, S.; Mari, E.; Migliorini, M.; Cherubini, C.; Trapani, S.; Zanoni, B.; Vincenzini, M. Investigation on microbiology of olive oil extraction process. Ital. J. Food Sci. 2015, 27, 237. [Google Scholar]
- Koidis, A.; Triantafillou, E.; Boskou, D. Endogenous microflora in turbid virgin olive oils and the physicochemical characteristics of these oils. Eur. J. Lipid Sci. Technol. 2008, 110, 164–171. [Google Scholar] [CrossRef]
- Guerrini, L.; Masella, P.; Migliorini, M.; Cherubini, C.; Parenti, A. Addition of a steel pre-filter to improve plate filter-press performance in olive oil filtration. J. Food Eng. 2015, 157, 84–87. [Google Scholar] [CrossRef]
- Breschi, C.; Guerrini, L.; Domizio, P.; Ferraro, G.; Calamai, L.; Canuti, V.; Masella, P.; Parenti, A.; Fratini, E.; Fia, G.; et al. Physical, Chemical, and Biological Characterization of Veiled Extra virgin Olive Oil Turbidity for Degradation Risk Assessment. Eur. J. Lipid Sci. Technol. 2019, 121, 1900195. [Google Scholar] [CrossRef]
- Ciafardini, G.; Zullo, B.A. Microbiological activity in stored olive oil. Int. J. Food Microbiol. 2002, 75, 111–118. [Google Scholar] [CrossRef]
- Ciafardini, G.; Zullo, B.A. Effect of lipolytic activity of Candida adriatica, Candida diddensiae and Yamadazyma terventina on the acidity of extra-virgin olive oil with different polyphenol and water content. Food Microbiol. 2015, 47, 12–20. [Google Scholar] [CrossRef]
- Zullo, B.A.; Cioccia, G.; Ciafardini, G. Effects of some oil-born yeasts on the sensory characteristics of Italian virgin olive oil during its storage. Food Microbiol. 2013, 36, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Fregapane, G.; Lavelli, V.; León, S.; Kapuralin, J.; Desamparados Salvador, M. Effect of filtration on virgin olive oil stability during storage. Eur. J. Lipid Sci. Technol. 2006, 108, 134–142. [Google Scholar] [CrossRef]
- Fortini, M.; Migliorini, M.; Cherubini, C.; Cecchi, L.; Guerrini, L.; Masella, P.; Parenti, A. Shelf life and quality of olive oil filtered without vertical centrifugation. Eur. J. Lipid Sci. Technol. 2016, 118, 1213–1222. [Google Scholar] [CrossRef]
- Jabeur, H.; Zribi, A.; Bouaziz, M. Changes in chemical and sensory characteristics of Chemlali extra-virgin olive oil as depending on filtration. Eur. J. Lipid Sci. Technol. 2017, 119, 1500602. [Google Scholar] [CrossRef]
- European Union Commission. Regulation EEC 1989/2003 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Euro. Comm. L 2003, 295, 57–77. [Google Scholar]
- Fortini, M.; Migliorini, M.; Cherubini, C.; Cecchi, L.; Calamai, L. Multiple internal standard normalization for improving HS-SPME-GC-MS quantitation in virgin olive oil volatile organic compounds (VOO-VOCs) profile. Talanta 2017, 165, 641–652. [Google Scholar] [CrossRef]
- Zullo, B.A.; Ciafardini, G. Changes in Physicochemical and Microbiological Parameters of Short and Long-Lived Veiled (Cloudy) Virgin Olive Oil Upon Storage in the Dark. Eur. J. Lipid Sci. Technol. 2018, 120, 1700309. [Google Scholar] [CrossRef]
- Zullo, B.A.; Cioccia, G.; Ciafardini, G. Distribution of dimorphic yeast species in commercial extra virgin olive oil. Food Microbiol. 2010, 27, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- International Olive Council. IOC/T.20/Doc. No 22—Organoleptic Assessment of Extra Virgin Olive Oil Applying to Use a Designation of Origin. Madrid, Spain. 2018.
- International Olive Council. IOC/T.20/Doc. No 14—Guide for the Selection, Training and Quality Control of Virgin Olive Oil Tasters-Qualifications of Tasters, Panel Leaders and Trainers. Madrid, Spain. 2020.
- Dunn, P.K.; Smyth, G.K. Generalized Linear Models with Examples in R; Springer: New York, NY, USA, 2018. [Google Scholar]
- Derossi, A.; Severini, C.; Cassi, D. Mass transfer mechanisms during dehydration of vegetable food: Traditional and innovative approaches. In Advanced Topics in Mass Transfer; IntechOpen: London, UK, 2011. [Google Scholar]
- Mossel, D.A.A.; Corry, J.E.L.; Strujik, C.B.; Baird, R.M. Essential of the Microbiology of Foods: A Textbook for Advanced Studies; John Wiley & Sons Ltd.: New York, NY, USA, 1995. [Google Scholar]
- Gila, A.; Beltrán, G.; Bejaoui, M.A.; Aguilera, M.P.; Jiménez, A. How clarification systems can affect virgin olive oil composition and quality at industrial scale. Eur. J. Lipid Sci. Technol. 2017, 119, 1600479. [Google Scholar] [CrossRef]
- Ciafardini, G.; Zullo, B.A. Virgin olive oil yeasts: A review. Food Microbiol. 2018, 70, 245–253. [Google Scholar] [CrossRef] [PubMed]
- García-Vico, L.; Belaj, A.; Sánchez-Ortiz, A.; Martínez-Rivas, J.M.; Pérez, A.G.; Sanz, C. Volatile compound profiling by HS-SPME/GC-MS-FID of a core olive cultivar collection as a tool for aroma improvement of virgin olive oil. Molecules 2017, 22, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angerosa, F.; Mostallino, R.; Basti, C.; Vito, R. Virgin olive oil odour notes: Their relationships with volatile compounds from the lipoxygenase pathway and secoiridoid compounds. Food Chem. 2000, 68, 283–287. [Google Scholar] [CrossRef]
- Bubola, K.B.; Koprivnjak, O.; Sladonja, B. Influence of filtration on volatile compounds and sensory profile of virgin olive oils. Food Chem. 2012, 132, 98–103. [Google Scholar] [CrossRef]
- Morales, M.T.; Alonso, M.V.; Rios, J.J.; Aparicio, R. Virgin olive oil aroma: Relationship between volatile compounds and sensory attributes by chemometrics. J. Agric. Food Chem. 1995, 43, 2925–2931. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Mattei, A.; Rovellini, P.; Zunin, P. Effect of membrane filtration on the flavor of virgin olive oil. Eur. J. Lipid Sci. Technol. 2008, 110, 1109–1115. [Google Scholar] [CrossRef]
- Dickinson, J.R.; Salgado, L.E.; Hewlins, M.J. The catabolism of amino acids to long chain and complex alcohols in Saccharomyces cerevisiae. J. Biol. Chem. 2003, 278, 8028–8034. [Google Scholar] [CrossRef] [Green Version]
- Mahadevan, K.; Farmer, L. Key odor impact compounds in three yeast extract pastes. J. Agric. Food Chem. 2006, 54, 7242–7250. [Google Scholar] [CrossRef]
- Loscos, N.; Hernandez-Orte, P.; Cacho, J.; Ferreira, V. Release and formation of varietal aroma compounds during alcoholic fermentation from nonfloral grape odorless flavor precursors fractions. J. Agric. Food Chem. 2007, 55, 6674–6684. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, L.; Migliorini, M.; Giusti, M.; Parenti, A. The influence of crusher speed on extra virgin olive oil characteristics. Eur. J. Lipid Sci. Technol. 2017, 119, 1600156. [Google Scholar] [CrossRef]
- Aparicio Ruiz, R.; Tena Pajuelo, N.; Romero del Río, I.; García González, D.L.; Morales Millán, M.T. Predicting extra virgin olive oil freshness during storage by fluorescence spectroscopy. Grasas Aceites 2017, 68, e219. [Google Scholar] [CrossRef] [Green Version]
- Kanavouras, A.; Hernandez-Münoz, P.; Coutelieris, F.; Selke, S. Oxidation-derived flavor compounds as quality indicators for packaged olive oil. J. Am. Oil Chem. Soc. 2004, 81, 251. [Google Scholar] [CrossRef]
- Oueslati, I.; Krichene, D.; Manaï, H.; Taamalli, W.; Zarrouk, M.; Flamini, G. Monitoring the volatile and hydrophilic bioactive compounds status of fresh and oxidized Chemlali virgin olive oils over olive storage times. Food Res. Int. 2018, 112, 425–433. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, S.C.; Shoemaker, C.F. Volatile constituents in sensory defective virgin olive oils. Flavour Frag. J. 2016, 31, 22–30. [Google Scholar] [CrossRef]
- Elsorady, M.E.I.; Girgis, A.Y.; El-labban, A.A. Influence of filtration on olive oil quality during storage. Life Sci. J. 2017, 14, 17–26. [Google Scholar]
- Brkić Bubola, K.; Lukić, M.; Mofardin, I.; Butumović, A.; Koprivnjak, O. Filtered vs. naturally sedimented and decanted virgin olive oil during storage: Effect on quality and composition. LWT—Food Sci. Technol. 2017, 84, 370–377. [Google Scholar] [CrossRef]
- Sacchi, R.; Caporaso, N.; Paduano, A.; Genovese, A. Industrial-scale filtration affects volatile compounds in extra virgin olive oil cv. Ravece. Eur. J. Lipid Sci. Technol. 2015, 117, 2007–2014. [Google Scholar] [CrossRef]





| F | V | ||||
|---|---|---|---|---|---|
| t0 | t1 | t0 | t1 | Legal Limits for “Extra Virgin” Category [5,6] | |
| Acidity (% oleic acid) | 0.35 ± 0.09 ax | 0.36 ± 0.10 ax | 0.32 ± 0.02 ax | 0.33 ± 0.03 ax | ≤0.8 |
| Peroxide value (meqO2kg−1) | 6.2 ± 0.7 bx | 6.2 ± 0.8 bx | 5.8 ± 0.3 ax | 5.9 ± 0.4 ax | ≤20 |
| K232 | 1.59 ± 0.06 ax | 1.60 ± 0.05 ax | 1.59 ± 0.07 ax | 1.62 ± 0.07 ax | ≤2.50 |
| K270 | 0.09 ± 0.01 bx | 0.09 ± 0.02 bx | 0.18 ± 0.01 ax | 0.18 ± 0.01 ax | ≤0.22 |
| ∆K | −0.004 ± 0.000 ay | −0.001 ± 0.002 ax | −0.004 ± 0.001 ax | −0.003 ± 0.003 ax | ≤0.01 |
| Microbial cell count (log CFU/g) | 0.6 ± 1.0 bx | 0.0 ± 0.0 bx | 3.8 ± 0.2 ax | 3.5 ± 0.3 ax | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrini, L.; Breschi, C.; Zanoni, B.; Calamai, L.; Angeloni, G.; Masella, P.; Parenti, A. Filtration Scheduling: Quality Changes in Freshly Produced Virgin Olive Oil. Foods 2020, 9, 1067. https://doi.org/10.3390/foods9081067
Guerrini L, Breschi C, Zanoni B, Calamai L, Angeloni G, Masella P, Parenti A. Filtration Scheduling: Quality Changes in Freshly Produced Virgin Olive Oil. Foods. 2020; 9(8):1067. https://doi.org/10.3390/foods9081067
Chicago/Turabian StyleGuerrini, Lorenzo, Carlotta Breschi, Bruno Zanoni, Luca Calamai, Giulia Angeloni, Piernicola Masella, and Alessandro Parenti. 2020. "Filtration Scheduling: Quality Changes in Freshly Produced Virgin Olive Oil" Foods 9, no. 8: 1067. https://doi.org/10.3390/foods9081067